Dipicolinic acid
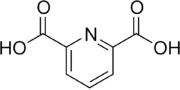
Dipicolinic acid (pyridine-2,6-dicarboxylic acid or PDC and DPA) is a chemical compound which plays a role in the heat resistance of bacterial endospores. It is also used to prepare dipicolinato ligated lanthanide and transition metal complexes for ion chromatography.[1]
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Pyridine-2,6-dicarboxylic acid | |
| Other names
2,6-Pyridinedicarboxylic acid | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.007.178 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C7H5NO4 | |
| Molar mass | 167.120 g·mol−1 |
| Melting point | 248 to 250 °C (478 to 482 °F; 521 to 523 K) |
| Hazards | |
| Main hazards | Irritant (Xi) |
| R-phrases (outdated) | R36/37/38 |
| S-phrases (outdated) | S26 S36 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Biological role
Dipicolinic composes 5% to 15% of the dry weight of bacterial spores.[2][3] It has been implicated as responsible for the heat resistance of the endospore,[2][4] although mutants resistant to heat but lacking dipicolinic acid have been isolated, suggesting other mechanisms contributing to heat resistance are at work.[5] Two genera of bacterial pathogens are known to produce endospores: the aerobic Bacillus and anaerobic Clostridium.[6]
Dipicolinic acid forms a complex with calcium ions within the endospore core. This complex binds free water molecules, causing dehydration of the spore. As a result, the heat resistance of macromolecules within the core increases. The calcium-dipicolinic acid complex also functions to protect DNA from heat denaturation by inserting itself between the nucleobases, thereby increasing the stability of DNA.[7]
The high concentration of DPA in and specificity to bacterial endospores has long made it a prime target in analytical methods for the detection and measurement of bacterial endospores. A particularly important development in this area was the demonstration by Rosen et al. of an assay for DPA based on photoluminescence in the presence of terbium,[8] although this phenomenon was first investigated for using DPA in an assay for terbium by Barela and Sherry.[9] Extensive subsequent work by numerous scientists has elaborated on and further developed this approach.
Environmental behavior
Simple substituted pyridines vary significantly in environmental fate characteristics, such as volatility, adsorption, and biodegradation.[10] Dipicolinic acid is among the least volatile, least adsorbed by soil, and most rapidly degraded of the simple pyridines.[11] A number of studies have confirmed dipicolinic acid is biodegradable in aerobic and anaerobic environments, which is consistent with the widespread occurrence of the compound in nature.[12] With a high solubility (5g/liter) and limited sorption (estimated Koc = 1.86), utilization of dipicolinic acid as a growth substrate by microorganisms is not limited by bioavailability in nature.[13]
See also
- Dinicotinic acid, an isomeric dicarboxylic acid
References
- 2,6-Pyridinedicarboxylic acid at Sigma-Aldrich
- Sliemandagger, TA.; Nicholson, WL. (2001). "Role of Dipicolinic Acid in Survival of Bacillus subtilis Spores Exposed to Artificial and Solar UV Radiation". Applied and Environmental Microbiology. 67 (3): 1274–1279. doi:10.1128/aem.67.3.1274-1279.2001. PMC 92724. PMID 11229921.
- Sci-Tech Dictionary. McGraw-Hill Dictionary of Scientific and Technical Terms, McGraw-Hill Companies, Inc.
- Madigan, M., J Martinko, J. Parker (2003). Brock Biology of Microorganisms, 10th edition. Pearson Education, Inc., ISBN 981-247-118-9.
- Prescott, L. (1993). Microbiology, Wm. C. Brown Publishers, ISBN 0-697-01372-3.
- Gladwin, M. (2008). Clinical Microbiology Made Ridiculously Simple, MedMaster, Inc., ISBN 0-940780-81-X.
- Madigan. M, Martinko. J, Bender. K, Buckley. D, Stahl. D, (2014), Brock Biology of Microorganisms, 14th Edition, p. 78, Pearson Education Inc., ISBN 978-0-321-89739-8.
- Rosen, D.L.; Sharpless, C.; McGown, L.B. (1997). "Bacterial Spore Detection and Determination by Use of Terbium Dipicolinate Photoluminescence". Analytical Chemistry. 69 (6): 1082–1085. doi:10.1021/ac960939w.
- Barela, T.D.; Sherry, A.D. (1976). "A simple, one step fluorometric method for determination of nanomolar concentrations of terbium". Analytical Biochemistry. 71 (2): 351–357. doi:10.1016/s0003-2697(76)80004-8.
- Sims, G. K.; O'Loughlin, E.J. (1989). "Degradation of pyridines in the environment". CRC Critical Reviews in Environmental Control. 19 (4): 309–340. doi:10.1080/10643388909388372.
- Sims, G. K.; Sommers, L.E. (1986). "Biodegradation of pyridine derivatives in soil suspensions". Environmental Toxicology and Chemistry. 5 (6): 503–509. doi:10.1002/etc.5620050601.
- Ratledge, Colin (ed). 2012. Biochemistry of microbial degradation. Springer Science and Business Media Dordrecht, Netherlands. 590 pages . doi:10.1007/978-94-011-1687-9
- Anonymous. MSDS. pyridine-2-6-carboxylic-acid .Jubilant Organosys Limited. http://www.jubl.com/uploads/files/39msds_msds-pyridine-2-6-carboxylic-acid.pdf
External links
- JPL Develops High-Speed Test to Improve Pathogen Decontamination at JPL.
- Spotting Spores at Astrobiology Magazine.