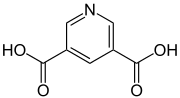
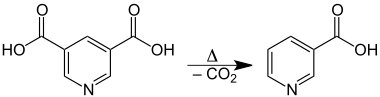Dinicotinic acid
Dinicotinic acid (pyridine-3,5-dicarboxylic acid) is an organic compound that belongs to the heterocycles (more precisely, heteroaromatics). It belongs to the group of pyridine dicarboxylic acids and consists of a pyridine ring, which carries two carboxy groups in the 3- and 5-position.
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Pyridine-3,5-dicarboxylic acid | |
| Other names
3,5-Pyridinedicarboxylic acid | |
| Identifiers | |
3D model (JSmol) |
|
| 131640 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.007.177 |
| EC Number |
|
| 279307 | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C7H5NO4 | |
| Molar mass | 167.120 g·mol−1 |
| Hazards | |
| GHS pictograms |  |
| GHS Signal word | Warning |
GHS hazard statements |
H315, H319, H335 |
| P261, P264, P271, P280, P302+352, P304+340, P305+351+338, P312, P321, P332+313, P337+313, P362, P403+233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Upon heating, it decarboxylates and decomposes to nicotinic acid:[1]
References
- Alam, Mahbub; Khan, M. Hafeez (1980). "Preparation of some nicotinic acid derivatives". Philippine Journal of Science. 109 (1–2): 19–21.CS1 maint: multiple names: authors list (link)
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.