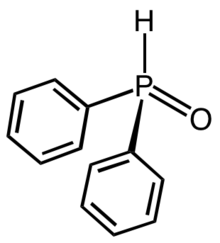
Diphenylphosphine oxide
Diphenylphosphine oxide is an organophosphorus compound with the formula (C6H5)2P(O)H. It is a white solid that soluble in polar organic solvents. The compound is used in Buchwald-Hartwig coupling reactions to introduce a diphenylphosphino substituent.[1] Analogous to the behavior of phosphorous acid, diphenylphosphine oxide exists in equilibrium with a minor tautomer hydroxydiphenylphosphine (CAS#24630-80-6) (C6H5)2POH.
 | |
| Identifiers | |
|---|---|
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| |
| |
| Properties | |
| C12H11OP | |
| Molar mass | 202.19 |
| Appearance | white solid |
| Melting point | 56-57 ºC |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Synthesis
Diphenylphosphine oxide can be prepared by the reaction of phosphonic esters, such as diethylphosphite, with Grignard reagents. Alternatively, it may be prepared by the partial hydrolysis of chlorodiphenylphosphine[1] or diphenylphosphine.[2]
Reactions
Organophosphinous acids are deoxygenated with DIBAH. The resulting secondary phosphines are precursors to phosphine ligands.[3]
References
- Jeffrey O. Saunders, Zheng Wang, Kuiling Ding "Diphenylphosphine Oxide" Encyclopedia of Reagents for Organic Synthesis, 2007 John Wiley & Sons, Ltd.doi:10.1002/047084289X.rd428.pub2
- RAUHUT, M. M.; CURRIER, HELEN A. (November 1961). "Oxidation of Secondary Phosphines to Secondary Phosphine Oxides". The Journal of Organic Chemistry. 26 (11): 4626–4628. doi:10.1021/jo01069a102.
- Carl A. Busacca, Jon C. Lorenz, Paul Sabila, Nizar Haddad, Chris H. Senanyake (2007). "Synthesis Of Electron-Deficient Secondary Phosphine Oxides And Secondary Phosphines: Bis[3,5-bis(Trifluoromethyl)phenyl]phosphine Oxide And Bis[3,5-bis(Trifluoromethyl)phenyl]phosphine". Org. Synth. 84: 242. doi:10.15227/orgsyn.084.0242.CS1 maint: uses authors parameter (link)