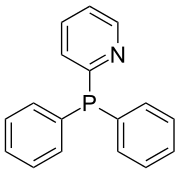
Diphenyl-2-pyridylphosphine
Diphenyl-2-pyridylphosphine is an organophosphorus compound with the formula P(C6H5)2(2-C5H4N). It is the most widely used mono-pyridylphosphine ligand.[1] Other mono-pyridylphosphines ligands (3-, 4-) are not common in chemical literature; however, tris-pyridylphosphines have been thoroughly investigated as ligands in transition metal complexes used for catalysis. Pyridylphosphines, including diphenyl-2-pyridylphosphine, may bind transition metals as monodentate or bidentate ligands4. Diphenyl-2-pyridylphosphine behaves as a P-bound monodentate ligand, or a P,N-bound bidentate ligand. Diphenyl-2-pyridylphosphine is a sought after ligand for its ability to relay protons to transition metals such as palladium(II) in homogeneous catalysis.[2]
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Diphenyl-2-pyridylphosphine | |
| Other names
2-(Diphenylphosphino)-pyridine | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.157.265 |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C17H14NP | |
| Molar mass | 263.280 g·mol−1 |
| Appearance | White crystalline solid |
| Melting point | 85 °C (185 °F; 358 K) |
| Boiling point | 163 °C (325 °F; 436 K) |
| Hazards | |
| Main hazards | GHS07
Acute toxicity (oral, dermal, inhalation), category 4 Skin irritation, category 2 Eye irritation, category 2 Skin sensitization, category 1 Specific Target Organ Toxicity – Single exposure, category 3 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Synthesis
Diphenyl-2-pyridylphosphine is prepared from 2-lithiopyridine with chlorodiphenylphosphine:[3]
Diphenyl-2-pyridylphosphine is an integral ligand in the Pd(II) catalyzed carbonylation of alkynes. The pi-donor ability of one bidentate P,N-coordinated ligand is highly stabilizing to the metal center.[4] While a second monodentate, N-protonated ligand transfers protons to the metal to be used in catalysis.[4] The role of the pyridyl group in this catalytic cycle is evident when the ligand is replaced by triphenyl phosphine, and rates of catalysis a greatly decreased. This catalytic process is an important step in the production of polymers, and other fine chemicals.[3]
RC2H + CO + XH + Pd cat → RCCH2COX + RCHCHCOX Pd cat = Pd(OAc)2/Ph2PPy/ CH3SO3H R=alkyl, aryl X=OH, OR’, NR2’ Scheme 1: Carbonylation of alkynes by cationic Pd(II) catalyst with a diphenyl-2-pyridylphosphine ligand.
References
- Kluwer, Alexander M.; Ahmad, Irshad; Reek, Joost N.H. (2007). "Improved synthesis of monodentate and bidentate 2- and 3-pyridylphosphines". Tetrahedron Letters. 48 (17): 2999. doi:10.1016/j.tetlet.2007.02.127.
- Drent, E.; Arnoldy, P.; Budzelaar, P.H.M. (1994). "Homogeneous catalysis by cationic palladium complexes. Precision catalysis in the carbonylation of alkynes". Journal of Organometallic Chemistry. 475: 57. doi:10.1016/0022-328X(94)84007-5.
- BrüCk, Andreas; Ruhland, Klaus (2009). "Investigation of the Dynamic Solution Behavior of Chloro(diene)rhodium(I) Phosphine Complexes with a Pendant Unsaturated Heterocycle at Phosphorus (2-pyridyl, 2-imidazyl; diene = COD, NBD)". Organometallics. 28 (22): 6383. doi:10.1021/om900324a.
- Doherty, S; Knight, J; Bentham, M. (2006). "The first insoluble polymer-bound palladium complexes of 2-pyridyldiphenylphosphine: Highly efficient catalysts for the alkoxycarbonylation of terminal alkynes". Chem. Commun.: 88–90. doi:10.1039/b512556a.
Further reading
- Scrivanti, A.; Bertoldini, M.; Beghetto, V.; Matteoli, U.; Venzo, A. (2009). "Protonation of palladium(II)-allyl and palladium(0)-olefin complexes containing 2-pyridyldiphenylphosphine". Journal of Organometallic Chemistry. 694: 131. doi:10.1016/j.jorganchem.2008.09.063.