Dimer acid
Dimer acids, or dimerized fatty acids, are dicarboxylic acids prepared by dimerizing unsaturated fatty acids obtained from tall oil, usually on clay catalysts. The CAS number of the material is [61788-89-4]. Dimer acids are used primarily for synthesis of polyamide resins and polyamide hot melt adhesives. They are also used in alkyd resins, adhesives, surfactants, as fuel oil additives, lubricants, etc.[1] It is a light yellow or yellow viscous transparent liquid. It has been described as non-toxic but research now shows this and other PFAS chemicals, also known as 'forever chemicals' are in fact highly toxic and undergoing EPA and other regulatory review as well as investigation by the North Carolina Attorney General and others. https://spectrumlocalnews.com/nc/triangle-sandhills/news/2020/08/13/chemical-company-agrees-to-clean-pfas-leaks-in-cape-fear-river
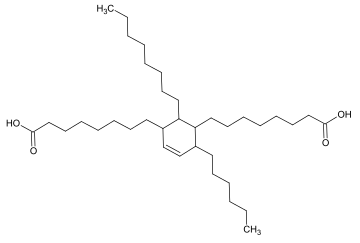
Dimer acid usually contains predominantly a dimer of stearic acid. It is also called C36 dimer acid.[2]
Trimer acid is a corresponding material where the resulting molecule consists of three fatty acid molecules. Its CAS number is [68937-90-6].
Dimer acids can be converted to dimer amines by reaction with ammonia and subsequent reduction.
Production
Dimer fatty acids are produced from different fatty acids by heating. Necessary are a fatty acid with conjugated double bonds or other unsaturated fatty acids. Examples of such fatty acids are conjugated linoleic acids. The reaction is carried out via Diels-Alder addition, whereby a partially unsaturated C6 ring is formed.[3] Besides the dimer, trimers as well as (unreacted) monomers of the fatty acids may be present in the mixture.
References
- Archived December 4, 2008, at the Wayback Machine
- C36 Dimer acid | 61788-89-4
- Walter Krauß (1998), Bindemittel für lösemittelhaltige und lösemittelfreie Systeme (in German) (2., erw. und neubearb. ed.), Stuttgart u. a.: Hirzel, p. 186, ISBN 3-7776-0886-6