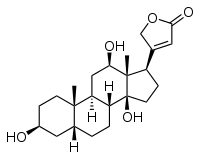
Digoxigenin
Digoxigenin (DIG) is a steroid found exclusively in the flowers and leaves of the plants Digitalis purpurea, Digitalis orientalis and Digitalis lanata (foxgloves), where it is attached to sugars, to form the glycosides (e.g. Lanatoside C).[2]
 | |
| Names | |
|---|---|
| IUPAC name
3-[(3S,5R,8R,9S,10S,12R,13S,14S,17R)-3,12,14-trihydroxy-10,13-dimethyl-1,2,3,4,5,6,7,8,9,11,12,15,16,17-tetradecahydrocyclopenta[a]phenanthren-17-yl]-2H-furan-5-one | |
| Identifiers | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.015.279 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| Properties | |
| C23H34O5 | |
| Molar mass | 390.51 g/mol |
| log P | 2.57510[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Use in biotechnology
Digoxigenin is a hapten, a small molecule with high antigenicity, that is used in many molecular biology applications similarly to other popular haptens such as 2,4-Dinitrophenol, biotin, and fluorescein. Typically, digoxigenin is introduced chemically (conjugation) into biomolecules (proteins, nucleic acids) to be detected in further assays.
DIG-binding proteins. Tinberg et al. designed artificial proteins that bind DIG. Their best binder, DIG10.3, was a 141 amino acid protein that bound DIG with a dissociation constant (Kd) of 541 (+/- 193) pM.[3]
Anti-digoxigenin antibodies with high affinities and specificity are used in a variety of biological immuno-assays (e.g. ELISA). The antibodies are labeled with dyes, enzymes or fluorescence, directly or secondarily, for visualization and detection.
Digoxigenin is thus an all-purpose immuno-tag, and in particular a standard immunohistochemical marker for in situ hybridization.[4][5] In this case it is conjugated to a single species of RNA nucleoside triphosphate (typically uridine), which is then incorporated into RNA (a "riboprobe") as it is synthesized by the cellular machinery.
It allows to make :
- sensitive non-radioactive in situ hybridization probes to detect nucleic acids in plants, able to detect 1 µg of plasmid DNA.[6]
- peptide-DIG conjugates, i.e. bradykinin assay by very sensitive chemiluminescence immunoassays.[7]
- fluorescent and DIG-labeled tracers for competitive immunoassays, i.e. to limit detect digoxin, a drug used to cure cardiac arrhythmia, down to 0.2 ng mL−1.[8]
- Digoxigenin may be conjugated to sugars to study glycosylation events,[9] even in biological systems.
References
- "Digoxigenin". Material Data Safety Sheet. ChemSrc.
- Polya G (2003). Biochemical Targets of Plant Bioactive Compounds. New York: CRC Press. ISBN 978-0415308298.
- Tinberg CE, Khare SD, Dou J, Doyle L, Nelson JW, Schena A, Jankowski W, Kalodimos CG, Johnsson K, Stoddard BL, Baker D (September 2013). "Computational design of ligand-binding proteins with high affinity and selectivity". Nature. 501 (7466): 212–216. doi:10.1038/nature12443. PMC 3898436. PMID 24005320.
- Eisel D, Grünewald-Janho S, Krushen B, eds. (2002). DIG Application Manual for Nonradioactive in situ Hybridization (3rd ed.). Penzberg: Roche Diagnostics.
- Hauptmann G, Gerster T (August 1994). "Two-color whole-mount in situ hybridization to vertebrate and Drosophila embryos". Trends in Genetics. 10 (8): 266. doi:10.1016/0168-9525(90)90008-T. PMID 7940754.
- Hart SM, Basu C (April 2009). "Optimization of a digoxigenin-based immunoassay system for gene detection in Arabidopsis thaliana" (pdf). Journal of Biomolecular Techniques. 20 (2): 96–100. PMC 2685603. PMID 19503620.
- Décarie A, Drapeau G, Closset J, Couture R, Adam A (1994). "Development of digoxigenin-labeled peptide: application to chemiluminoenzyme immunoassay of bradykinin in inflamed tissues". Peptides. 15 (3): 511–8. doi:10.1016/0196-9781(94)90214-3. PMID 7937327.
- Mayilo S, Ehlers B, Wunderlich M, Klar TA, Josel HP, Heindl D, Nichtl A, Kürzinger K, Feldmann J (July 2009). "Competitive homogeneous digoxigenin immunoassay based on fluorescence quenching by gold nanoparticles". Analytica Chimica Acta. 646 (1–2): 119–22. doi:10.1016/j.aca.2009.05.023. PMID 19523564.
- Goodarzi MT, Rafiq M, Turner G (May 1995). "An improved multiwell immunoassay using digoxigenin-labelled lectins to study the glycosylation of purified glycoproteins". Biochemical Society Transactions. 23 (2): 168S. doi:10.1042/bst023168s. PMID 7672194.