Dichlorofluoromethane
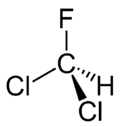
Dichlorofluoromethane or Freon 21 or R 21 is a halomethane or hydrochlorofluorocarbon with the formula CHCl2F. It is a colorless and odorless gas. It is produced by fluorination of chloroform using a catalyst such as antimony trifluoride:[4]
- CHCl3 + HF → CHCl2F
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Dichloro(fluoro)methane | |
| Other names
Dichlorofluoromethane Fluorodichloromethane Monofluorodichloromethane Dichloromonofluoromethane Freon 21 Refrigerant 21 R 21 HCFC 21 Algofrene Type 5 Arcton 7 Halon 112 UN 1029 Genetron 21 | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.000.791 |
| EC Number |
|
PubChem CID |
|
| RTECS number |
|
| UNII | |
| UN number | 1029 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| CHCl2F | |
| Molar mass | 102.92 g/mol |
| Appearance | Colorless gas |
| Odor | ether-like[1] |
| Density | 1.405 g/cm3 at 9 °C
1.366 kg/m3 at 25 °C |
| Melting point | −135 °C (−211 °F; 138 K) |
| Boiling point | 8.92 °C (48.06 °F; 282.07 K) |
| 9.420 g/l at 30 °C | |
| log P | 1.55 |
| Vapor pressure | 160 kPa |
Henry's law constant (kH) |
0.19 mol.kg−1.bar−1 |
| -48.8·10−6 cm3/mol | |
| Thermal conductivity | 0.0086 W m−1 K−1 (300 K)[2] |
| Hazards | |
| GHS pictograms |   |
| GHS Signal word | Warning |
GHS hazard statements |
H280, H420 |
| P410+403, P502 | |
| Flash point | nonflammable [1] |
| 522 °C (972 °F; 795 K) | |
| Lethal dose or concentration (LD, LC): | |
LC50 (median concentration) |
>800,000 mg/m3 (mouse, 2 hr) 49,900 ppm (rat, 4 hr)[3] |
LCLo (lowest published) |
100,000 ppm (guinea pig, <1 hr) 100,000 ppm (mouse, <1 hr)[3] |
| NIOSH (US health exposure limits): | |
PEL (Permissible) |
TWA 1000 ppm (4200 mg/m3)[1] |
REL (Recommended) |
TWA 10 ppm (40 mg/m3)[1] |
IDLH (Immediate danger) |
5000 ppm[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Uses
Dichlorofluoromethane was used as a propellant and refrigerant. Due to its role in ozone depletion, dichlorofluoromethane has been largely phased out. It has ozone depletion potential 0.04. Production and consumption has been since 2004 reduced to 15% of level from 1989 and it is to be phased out in 2015 according to Montreal Protocol.
Pyrolysis of a mixture of dichlorofluoromethane and chlorofluoromethane gives hexafluorobenzene:[4]
- 3 CHCl2F + 3 CH2ClF → C6F6 + 9 HCl
Additional physical data
Its critical point is at 178.5 °C (451.7 K) and 5.17 MPa (51.7 bar). At temperatures from 5 K to 105 K it has one phase in the space group Pbca.
Safety
Its toxicity is comparable to that of chloroform. Its TLV is 10 ppm.[4]
References
- NIOSH Pocket Guide to Chemical Hazards. "#0197". National Institute for Occupational Safety and Health (NIOSH).
- Touloukian, Y.S., Liley, P.E., and Saxena, S.C. Thermophysical properties of matter - the TPRC data series. Volume 3. Thermal conductivity - nonmetallic liquids and gases. Data book. 1970.
- "Dichloromonofluoromethane". Immediately Dangerous to Life and Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- Dagani, M. J.; = Barda, H. J.; Benya, T. J.; Sanders, D. C. "Bromine Compounds". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a04_405.
External links
- International Chemical Safety Card 1106
- NIOSH Pocket Guide to Chemical Hazards. "#0197". National Institute for Occupational Safety and Health (NIOSH).
- Termochemistry data at chemnet.ru
- Entry at Air Gas Liquide Encyclopaedia