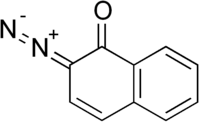
Diazonaphthoquinone
Diazonaphthoquinone (DNQ) is a diazo derivative of naphthoquinone. Upon exposure to light, DNQ converts to a derivative that is susceptible to etching. In this way, DNQ has become an important reagent in photoresist technology in the semiconductor industry.[1]
 | |
| Names | |
|---|---|
| IUPAC name
2-Diazo-2H-naphthalen-1-one | |
| Other names
1,2-Naphthoquinone diazide | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| |
| |
| Properties | |
| C10H6N2O | |
| Molar mass | 170.171 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Diazonaphthoquinone sulfonic acid esters are components of common photoresist materials. Such photoresists are used in the manufacture of semiconductors.[2][3][4] In this application DNQs are mixed with Novolac resin, a type of phenolic polymer. The DNQ functions as a dissolution inhibitor. During the masking/patterning process, portions of the photoresist film are exposed to light while others remain unexposed. In the unexposed regions of the resist film, the DNQ acts as a dissolution inhibitor and the resist remains insoluble in the aqueous base developer. In the exposed regions, the DNQ forms a ketene, which, in turn, reacts with ambient water to form a base soluble indene carboxylic acid. The exposed regions of the photoresist film become soluble in aqueous base; thus allowing the formation of a relief image during development.
Chemical reactions
Upon photolysis, DNQ undergoes a Wolff rearrangement to form a ketene. The ketene adds water to form indane-carboxylic acid.[5]
References
- Ralph Dammel (1993). Diazonaphthoquinone-based Resists. Int. Soc. Optical Engineering. ISBN 9780819410191.
- W. D. Hinsberg, G. M. Wallraff. (2005), "Lithographic Resists", Kirk-Othmer Encyclopedia of Chemical Technology, New York: John Wiley, doi:10.1002/0471238961.1209200808091419.a01.pub2, ISBN 9780471238966
- Chemical Information Review Document for Diazonaphthoquinone Derivatives Used in Photoresists, National Toxicology Program, January 2006
- Integrated Circuits: A Brief History
- N. C. de Lucas; J. C. Netto-Ferreira; J. Andraos; J. C. Scaiano (2001). "Nucleophilicity toward Ketenes: Rate Constants for Addition of Amines to Aryl Ketenes in Acetonitrile Solution". J. Org. Chem. 66 (5): 5016–5021. doi:10.1021/jo005752q. PMID 11463250. S2CID 12123114.