Depsipeptide
A depsipeptide is a peptide in which one or more of its amide, -C(O)NHR-, groups are replaced by the corresponding ester, -C(O)OR,[1] Many depsipeptides have both peptide and ester linkages. They are mainly found in marine and microbial natural products.[2]
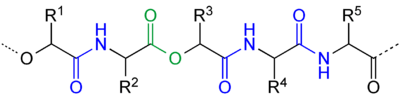
Depsipeptide natural products
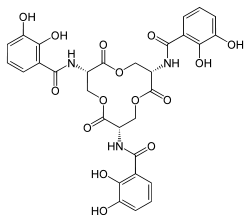
Several depsipeptides have been found to exhibit anti-cancer properties.[4]
A depsipeptide enzyme inhibitor includes romidepsin, a member of the bicyclic peptide class, a known histone deacetylase inhibitors (HDACi). It was first isolated as a fermentation product from Chromobacterium violaceum by the Fujisawa Pharmaceutical Company.[5]
Etamycin was shown in preliminary data in 2010 to have potent activity against MRSA in a mouse model.[6] Several depsipeptides from Streptomyces exhibit antimicrobial activity.[7][8] These form a new, potential class of antibiotics known as acyldepsipeptides (ADEPs). ADEPs target and activate the casein lytic protease (ClpP) to initiate uncontrolled peptide and unfolded protein degradation, killing many Gram-positive bacteria.[9][10][11]
Further reading
- papuamide Ford, PW; Gustafson, KR; McKee, TC; Shigematsu, N; Maurizi, LK; Pannell, LK; Williams, DE; de Silva, ED; Lassota, P; Allen, TM; Van Soest, R; Andersen, RJ; Boyd, MR (1999). "Papuamides A-D, HIV-Inhibitory and Cytotoxic Depsipeptides from the Sponges Theonella mirabilis and Theonella swinhoei Collected in Papua New Guinea". J. Am. Chem. Soc. 121: 5899–5909. doi:10.1021/ja990582o.
- neamphamide A Oku, N; Gustafson, KR; Cartner, LK; Wilson, JA; Shigematsu, N; Hess, S; Pannell, LK; Boyd, MR; McMahon, JB (2004). "Neamphamide A. A new HIV-inhibitory depsipeptide from the Papua New Guinea marine sponge Neamphius huxleyi". J. Nat. Prod. 67 (8): 1407–11. doi:10.1021/np040003f. PMID 15332865.
- callipeltin A Zampella, A; D'Auria, MV; Paloma, LG; Casapullo, A; Minale, L; Debitus, C; Henin, Y (1996). "Callipeltin A, an Anti-HIV Cyclic Depsipeptide from the New Caledonian Lithistida Sponge Callipelta sp.". J. Am. Chem. Soc. 118: 6202–9. doi:10.1021/ja954287p.
- mirabamides A-D Plaza, A; Gustchina, E; Baker, HL; Kelly, M; Bewley, CA (2007). "Mirabamides A-D. Depsipeptides from the sponge Siliquariaspongia mirabilis that inhibit HIV-1 fusion". J. Nat. Prod. 70 (11): 1753–60. doi:10.1021/np070306k. PMID 17963357.; Andjelic, CD; Planelles, V; Barrows, LR (2008). "Characterizing the Anti-HIV Activity of Papuamide A." Mar Drugs. 6 (4): 528–49. doi:10.3390/md20080027. PMC 2630844. PMID 19172193.
References
- IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "depsipeptides". doi:10.1351/goldbook.D01604
- Yasumasa Hamada, Takayuki Shioiri (2005). "Recent Progress of the Synthetic Studies of Biologically Active Marine Cyclic Peptides and Depsipeptides". Chem. Rev. 105: 4441–4482. doi:10.1021/cr0406312.CS1 maint: uses authors parameter (link)
- Walsh, Christopher T., Jun Liu, Frank Rusnak, and Masahiro Sakaitani (1990). "Molecular Studies on Enzymes in Chorismate Metabolism and the Enterobactin Biosynthetic Pathway". Chemical Reviews. 90 (7): 1105–1129. doi:10.1021/cr00105a003.CS1 maint: multiple names: authors list (link)
- Kitagaki, J.; Shi, G.; Miyauchi, S.; Murakami, S.; Yang, Y. (2015). "Cyclic depsipeptides as potential cancer therapeutics". Anticancer Drugs. 26: 259–71. doi:10.1097/CAD.0000000000000183. PMID 25419631.
- Yurek-George, Alexander; Cecil, Alexander Richard Liam; Mo, Alex Hon Kit; Wen, Shijun; Rogers, Helen; Habens, Fay; Maeda, Satoko; Yoshida, Minoru; et al. (2007). "The First Biologically Active Synthetic Analogues of FK228, the Depsipeptide Histone Deacetylase Inhibitor". Journal of Medicinal Chemistry. 50 (23): 5720–5726. doi:10.1021/jm0703800. PMID 17958342.
- Haste, Nina M; Perera, Varahenage R; Maloney, Katherine N; Tran, Dan N; Jensen, Paul; Fenical, William; Nizet, Victor; Hensler, Mary E (2010). "Activity of the streptogramin antibiotic etamycin against methicillin-resistant Staphylococcus aureus". Journal of Antibiotics. 63 (5): 219. doi:10.1038/ja.2010.22. PMC 2889693. PMID 20339399.
- K. H. Michel, R. E. Kastner (Eli Lilly and Company), US 4492650, 1985 [Chem. Abstr. 1985, 102, 130459]
- Osada, Hiroyuki; Yano, Tatsuya; Koshino, Hiroyuki; Isono, Kiyoshi (1991). "Enopeptin A, a novel depsipeptide antibiotic with anti-bacteriophage activity". The Journal of Antibiotics. 44 (12): 1463–1466. doi:10.7164/antibiotics.44.1463.
- Li; Him Shun, Dominic; Guarné, Alba; Maurizi, Michael R.; Cheng, Yi-Qiang; Wright, Gerard D.; Ghirlando, Rodolfo; Joseph, Ebenezer; Gloyd, Melanie; Seon Chung, Yu; Ortega, Joaquin (2010). "Acyldepsipeptide Antibiotics Induce The Formation Of A Structured Axial Channel In ClpP: A Model For The ClpX/ClpA-Bound State Of ClpP". Chemistry & Biology. 17 (9): 959–969. doi:10.1016/j.chembiol.2010.07.008. PMC 2955292. PMID 20851345.
- Hinzen, Berthold; Labischinski, Harald; Brötz-Oesterhelt, Heike; Endermann, Rainer; Benet-Buchholz, Jordi; Hellwig, Veronica; Häbich, Dieter; Schumacher, Andreas; Lampe, Thomas; Paulsen, Holger; Raddatz, Siegfried (2006). "Medicinal Chemistry Optimization of Acyldepsipeptides of the Enopeptin Class Antibiotics". ChemMedChem. 1 (7): 689–693. doi:10.1002/cmdc.200600055. PMID 16902918.
- Carney, Daniel W.; Schmitz, Karl R.; Truong, Jonathan V.; Sauer, Robert T.; Sello, Jason K. (2014). "Restriction of the Conformational Dynamics of the Cyclic Acyldepsipeptide Antibiotics Improves Their Antibacterial Activity". JACS. 136: 1922–1929. doi:10.1021/ja410385c.