Cyclooctatetraenide anion
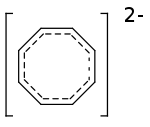
In chemistry, the cyclooctatetraenide anion or cyclooctatetraenide, more precisely cyclooctatetraenediide, is an aromatic species with a formula of [C8H8]2− and abbreviated as COT2−. It is the dianion of cyclooctatetraene. Salts of the cyclooctatetraenide anion can be stable, e.g., Dipotassium cyclooctatetraenide or disodium cyclooctatetraenide. More complex coordination compounds are known as cyclooctatetraenide complexes, such as the actinocenes.
 | |
| Names | |
|---|---|
| IUPAC name
cyclooctatetraenediide | |
| Identifiers | |
3D model (JSmol) |
|
| |
| Properties | |
| C8H82− | |
| Molar mass | 104.15 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
The structure is a planar symmetric octagon stabilized by resonance, meaning each atom bears a charge of –1⁄4. The length of the bond between carbon atoms is 1.432 Å. There are 10 π electrons.[1] The structure can serve as a ligand with various metals.
List of salts
| name | formula | CAS | remarks | references |
|---|---|---|---|---|
| Samarium(II) cyclooctatetraenide | Sm(C8H8) | [2] | ||
| Dipotassium samarium(II) cyclooctatetraenide | K2Sm(C8H8)2 | [2] | ||
| Neodymium cyclooctatetraenide | Nd(C8H8)2 | |||
| Terbium cyclooctatetraenide | Tb(C8H8)2 | |||
| protactinocene | (Pa(C8H8)2) | |||
| thorocene | (Th(C8H8)2) | |||
| Uranocene | U(C8H8)2 | |||
| neptunocene | (Np(C8H8)2) | |||
| plutonocene | (Pu(C8H8)2) | |||
gollark: <#348702212110680064>
gollark: I wouldn't. Base standard of living is... probably somewhat higher maybe, and there are more advancement opportunities.
gollark: a 🐝 b
gollark: However, gladiators who had nice stuff going on *were not the majority*.
gollark: Why should I have to CONSUME FOOD to CONTINUE EXISTING?
See also
- Tropylium ion
- Cyclopentadienyl anion
References
- Jug, Karl (November 1984). "Aromaticity in unusual heteropolar monocyclic rings with (4n + 2) π electrons". The Journal of Organic Chemistry. 49 (23): 4475–4478. doi:10.1021/jo00197a029.
- Wayda, Andrea L.; Cheng, Suzanne; Mukerji, Ishita (August 1987). "Cyclooctatetraenide derivatives of divalent samarium". Journal of Organometallic Chemistry. 330 (3): C17–C19. doi:10.1016/S0022-328X(00)99059-4.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.