Copper(I) thiophene-2-carboxylate
Copper(I) thiophene-2-carboxylate or CuTC is a thiophene and a reagent in organic chemistry that especially promotes the Ullmann reaction between aryl halides.[3]
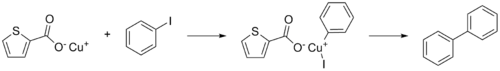 CuTC catalyzed Ullmann coupling
CuTC catalyzed Ullmann coupling
-thiophene-2-carboxylate.png) | |
| Names | |
|---|---|
| IUPAC name
Copper(I) thiophene-2-carboxylate | |
| Other names
CuTC | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.161.358 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C5H3CuO2S | |
| Molar mass | 190.68 g·mol−1 |
| Hazards | |
| Main hazards | Irritant |
| GHS pictograms |  |
| GHS Signal word | Warning |
GHS hazard statements |
H315, H319, H335 |
| P261, P264, P271, P280, P302+352, P304+340, P305+351+338, P312, P321, P332+313, P337+313, P362, P403+233, P405, P501 | |
| NIOSH (US health exposure limits): | |
PEL (Permissible) |
TWA 1 mg/m3 (as Cu)[2] |
REL (Recommended) |
TWA 1 mg/m3 (as Cu)[2] |
IDLH (Immediate danger) |
TWA 100 mg/m3 (as Cu)[2] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
References
- Copper(I) thiophene-2-carboxylate at Sigma-Aldrich
- NIOSH Pocket Guide to Chemical Hazards. "#0150". National Institute for Occupational Safety and Health (NIOSH).
- Shijie Zhang; Dawei Zhang; Lanny S. Liebeskind (1997). "Ambient Temperature, Ullmann-like Reductive Coupling of Aryl, Heteroaryl, and Alkenyl Halides". J. Org. Chem. 62 (8): 2312–2313. doi:10.1021/jo9700078. PMID 11671553.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.