Copper(II) thiocyanate
Copper(II) thiocyanate (or cupric thiocyanate) is a coordination polymer with formula Cu(SCN)2.[1] It is a black solid which slowly decomposes in moist air.[2] It was first reported in 1838 by Carl Ernst Claus and its structure was determined first in 2018.[3][1]
 Copper(II) thiocyanate | |
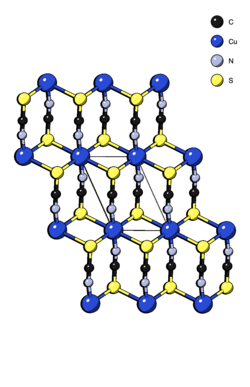 Crystal structure of copper(II) thiocyanate | |
| Names | |
|---|---|
| Other names
Cupric thiocyanate | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| |
| |
| Properties | |
| Cu(SCN)2 | |
| Molar mass | 179.71 g/mol[1] |
| Appearance | black powder |
| Density | 2.47 g/cm3[1] |
| Melting point | decomposes at 180 C [2] |
| 0.66·10−3 cm3/mol [1] | |
| Related compounds | |
Other anions |
Copper(II) bromide,Copper(II) chloride |
Other cations |
Copper(I) thiocyanate, Cobalt(II) thiocyanate, Mercury(II) thiocyanate, Ammonium thiocyanate Potassium thiocyanate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Structure
The structure of Cu(SCN)2 was determined via powder X-ray diffraction and consists of chains of Cu(NCS)2 linked together by weak Cu-S-Cu bonds into two-dimensional layers. It can be considered a Jahn-Teller distorted analogue of the mercury thiocyanate structure-type. Each copper is octahedrally coordinated by four sulfurs and two nitrogens. The sulfur end of the SCN- ligand is doubly bridging.[1]
Synthesis
Copper(II) thiocyanate can be prepared from the reaction of concentrated solutions of copper(II) and a soluble thiocyanate salt in water, precipitating as a black powder.[2][3] With rapid drying, pure Cu(SCN)2 can be isolated. Reaction at lower concentrations and for longer periods of time generates instead copper(I) thiocyanate.[4]
Magnetism
Copper(II) thiocyanate, like copper(II) bromide and copper(II) chloride, is a quasi low-dimensional antiferromagnet and it orders at 12K into a conventional Néel ground state.[1]
References
- Cliffe, Matthew J.; Lee, Jeongjae; Paddison, Joseph A. M.; Schott, Sam; Mukherjee, Paromita; Gaultois, Michael W.; Manuel, Pascal; Sirringhaus, Henning; Dutton, Siân E.; Grey, Clare P. (2018-04-25). "Low-dimensional quantum magnetism in Cu ( NCS ) 2 : A molecular framework material". Physical Review B. 97 (14): 144421. doi:10.1103/PhysRevB.97.144421. ISSN 2469-9950.
- Hunter, J. A.; Massie, W. H. S.; Meiklejohn, J.; Reid, J. (1969-01-01). "Thermal rearrangement in copper(II) thiocyanate". Inorganic and Nuclear Chemistry Letters. 5 (1): 1–4. doi:10.1016/0020-1650(69)80226-6. ISSN 0020-1650.
- Claus, C. (1838). "Beiträge zur näheren Kenntniss der Schwefelcyanmetalle". Journal für Praktische Chemie. 15 (1): 401–411. doi:10.1002/prac.18380150142. ISSN 1521-3897.
- Smith, D. L.; Saunders, V. I. (15 March 1982). "Preparation and structure refinement of the 2H polytype of β-copper(I) thiocyanate". Acta Crystallographica Section B Structural Crystallography and Crystal Chemistry. 38 (3): 907–909. doi:10.1107/S0567740882004361.