Civetone
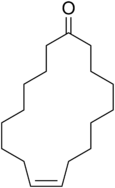
Civetone is a macrocyclic ketone and the main odorous constituent of civet oil.[1] It is a pheromone sourced from the African civet. It has a strong musky odor that becomes pleasant at extreme dilutions.[2] Civetone is closely related to muscone, the principal odoriferous compound found in musk; the structure of both compounds was elucidated by Leopold Ružička.[3] Today, civetone can be synthesized from precursor chemicals found in palm oil.[4]
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
(9Z)-Cycloheptadec-9-en-1-one | |||
| Other names
cis-Civetone; 9-Cycloheptadecen-1-one; Cycloheptadeca-9-en-1-one; (Z)-9-Cyclohepta-decen-1-one | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChemSpider | |||
| ECHA InfoCard | 100.008.013 | ||
| EC Number |
| ||
PubChem CID |
|||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C17H30O | |||
| Molar mass | 250.4195 | ||
| Appearance | Crystalline solid | ||
| Density | 0.917 at 33 °C | ||
| Melting point | 31 to 32 °C (88 to 90 °F; 304 to 305 K) | ||
| Boiling point | 342 °C (648 °F; 615 K) | ||
| Solubility in oils | soluble | ||
| Solubility in ethanol | soluble | ||
| Solubility in water | slightly soluble | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Uses
Civetone is used as a perfume fixative and flavor.
In order to attract jaguars to camera traps, field biologists have used the cologne Calvin Klein's Obsession For Men. It is believed that the civetone in the cologne resembles a territorial marking.[5]
gollark: Galaxtone, you really shouldn't have incited this instanity.
gollark: I have NO earphones!
gollark: Hi!
gollark: Okay, potatOS now has builtin `meta` support.
gollark: Mayhaps.
References
- The Merck Index, 15th Ed. (2013), p. 418, Monograph 2334, O'Neil: The Royal Society of Chemistry. Available online at: http://www.rsc.org/Merck-Index/monograph/mono1500002334
- Bedoukian, Paul Z. "Perfumery and Flavoring Synthetics", 2nd ed., p. 248, Elsevier, New York, 1967.
- Sell, Charles S. (1999). "Ingredients for the Modern Perfumery Industry". In Pybus, David H.; Sell, Charles S. (eds.). The Chemistry of Fragrances (1st ed.). Royal Society of Chemistry Publishing. pp. 51–124. ISBN 9780854045280.
- Yuen-May Choo, Kay-Eng Ooi and Ing-Hong Ooi (August 1994). "Synthesis of civetone from palm oil products". Journal of the American Oil Chemists' Society. Springer Berlin / Heidelberg. 71 (8): 911–913. doi:10.1007/bf02540473. ISSN 0003-021X.
- "You'll Never Guess How Biologists Lure Jaguars To Camera Traps". Scientific American Blog Network.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.