Cinchonine
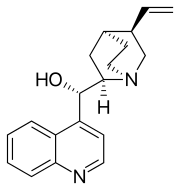
Cinchonine is an alkaloid found in Cinchona officinalis. It is used in asymmetric synthesis in organic chemistry. It is a stereoisomer and pseudo-enantiomer of cinchonidine.
 | |
| Names | |
|---|---|
| IUPAC name | |
| Systematic IUPAC name
(S)-[(2R,4S,5R)-5-ethenyl-1-azabicyclo[2.2.2]octan-2-yl](quinolin-4-yl)methanol | |
| Identifiers | |
3D model (JSmol) |
|
| 89689 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.003.850 |
| EC Number |
|
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C19H22N2O | |
| Molar mass | 294.39 g/mol |
| Melting point | 260-263 |
| Hazards | |
| GHS pictograms |  |
| GHS Signal word | Warning |
GHS hazard statements |
H302, H317, H332 |
| P261, P264, P270, P271, P272, P280, P301+312, P302+352, P304+312, P304+340, P312, P321, P330, P333+313, P363, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
References
- "Common Chemistry - Substance Details - 118-10-5 : Cinchonan-9-ol, (9S)-". commonchemistry.org. Retrieved 22 May 2020.
- IUPAC Chemical Nomenclature and Structure Representation Division (2013). "P-(Appendix 3, p. 1517)". In Favre, Henri A.; Powell, Warren H. (eds.). Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013. IUPAC–RSC. ISBN 978-0-85404-182-4.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.