Chan-Lam coupling
The Chan–Lam coupling reaction – also known as the Chan–Evans–Lam coupling is a cross-coupling reaction between an aryl boronic acid and an alcohol or an amine to form the corresponding secondary aryl amines or aryl ethers, respectively.[1] The Chan–Lam coupling is catalyzed by copper complexes. It can be conducted in air at room temperature. The more popular Buchwald–Hartwig coupling relies on the use of palladium.
| Chan-Lam coupling | |
|---|---|
| Named after | Dominic Chan Patrick Lam |
| Reaction type | Coupling reaction |
| Identifiers | |
| Organic Chemistry Portal | chan-lam-coupling |
| RSC ontology ID | RXNO:0000374 |
History
Chan, Evans, and Lam published their work nearly simultaneously.[2][3][4][5][6] the mechanism however remained mysterious for many years.
Mechanism
Analysis of the mechanism is complicated by the lability of copper reagents and the multicomponent nature of the reaction.[7] The reaction proceeds via the formation of copper-aryl complexes. A copper(III)-aryl-alkoxide or copper(III)-aryl-amide intermediate undergoes Reductive elimination to give the aryl ether or aryl amine, respectively:
- Ar-Cu(III)-NHR-L2 → Ar-NHR + Cu(I)L2
- Ar-Cu(III)-OR-L2 → Ar-OR + Cu(I)L2
Example
An example of the Chan–Lam coupling to synthesize biologically active compounds is shown below:
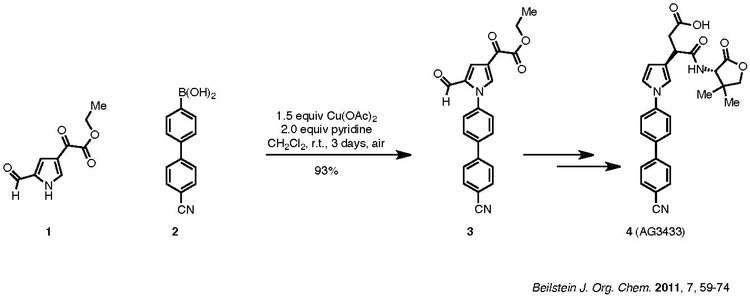 Reaction example of Chan–Lam coupling
Reaction example of Chan–Lam coupling
Compound 1, a pyrrole, is coupled with aryl boronic ester, 2, to afford product 3, which is then carried forward to the target 4. The nitrile group of 2 does not poison the catalyst. Pyridine is the ligand used for the reaction. Although the reaction requires three days, it was carried out at room temperature in ambient air and resulted in a 93% yield.
Further reading
- Kodepelly Sanjeeva Rao; Tian-Shung Wu (2012). "Chan-Lam coupling reactions: synthesis of heterocycles". Tetrahedron. 68 (38): 7735–7754. doi:10.1016/j.tet.2012.06.015.
References
- Jennifer X. Qiao, Patrick Y.S. Lam (2011). "Recent Advances in Chan–Lam Coupling Reaction: Copper-Promoted C–Heteroatom Bond Cross-Coupling Reactions with Boronic Acids and Derivatives". In Dennis G. Hall (ed.). Boronic Acids: Preparation and Applications in Organic Synthesis, Medicine and Materials. Wiley-VCH. pp. 315–361. doi:10.1002/9783527639328.ch6. ISBN 9783527639328.
- Chan, Dominic; Monaco, Kevin; Wang, R.; Winter, Michael (1998). "New N- and O-Arylations with Phenylboronic acids and Cupric Acetate". Tetrahedron Lett. 39 (19): 2933–2936. doi:10.1016/s0040-4039(98)00503-6.CS1 maint: uses authors parameter (link)
- Evans, David; Katz, J.; West, T. (1998). "Synthesis of Diaryl Ethers through the Copper-Promoted Arylation of Phenols with Arylboronic Acids. An Expedient Synthesis of Thyroxine". Tetrahedron Lett. 39 (19): 2937–2942. doi:10.1016/s0040-4039(98)00502-4.CS1 maint: uses authors parameter (link)
- Lam, Patrick; Clark, Charles; Saubern, Simon; Adams, Jessica; Winters, Michael; Chan, Dominic; Combs, Andrew (1998). "New Aryl/Heteroaryl C-N Bond Cross-coupling Reactions via Arylboronic Acid/Cupric Acetate Arylation". Tetrahedron Lett. 39: 2941–2944. doi:10.1016/s0040-4039(98)00504-8.CS1 maint: uses authors parameter (link)
- Lam, Patrick; Bonne, Damien; Vincent, Guillaume; Clark, Charles (2003). "Copper-promoted/catalyzed C-N and C-O Bond Cross-coupling with Vinylboronic Acid and Its Utilities". Tetrahedron Lett. 44: 4927–4931. doi:10.1016/s0040-4039(03)01037-2.CS1 maint: uses authors parameter (link)
- Chan, Dominic; Monaco, Kevin; Li, Renhua; Bonne, Damien; Clark, Charles; Lam, Patrick (2003). "Copper Promoted C-N and C-O Bond Cross-coupling with Phenyl and Pyridylboronates". Tetrahedron Lett. 44: 3863–3865. doi:10.1016/s0040-4039(03)00739-1.CS1 maint: uses authors parameter (link)
- Vantourout, J. C.; Miras, H. N.; Isidro-Llobet, A.; Sproules, S.; Watson, A. J. B. (2017). "Spectroscopic Studies of the Chan–Lam Amination: A Mechanism-Inspired Solution to Boronic Ester Reactivity" (PDF). Journal of the American Chemical Society. 139 (13): 4769–4779. doi:10.1021/jacs.6b12800. PMID 28266843.CS1 maint: uses authors parameter (link)