Cavernous hemangioma
Cavernous hemangioma, also called cavernous angioma, cavernoma, or cerebral cavernoma (CCM) (when referring to presence in the brain)[1][2] is a type of benign vascular tumor or hemangioma, where a collection of dilated blood vessels form a lesion. Because of this, blood flow through the cavities, or caverns, is slow. Additionally, the cells that form the vessels do not form the necessary junctions with surrounding cells. Also, the structural support from the smooth muscle is hindered, causing leakage into the surrounding tissue. It is the leakage of blood, known as a hemorrhage, from these vessels that causes a variety of symptoms known to be associated with this disease.
| Cavernous hemangioma | |
|---|---|
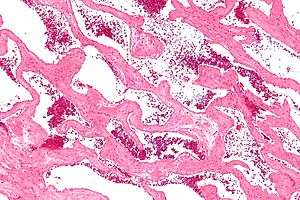 | |
| Micrograph of a cavernous liver hemangioma. H&E stain. | |
| Specialty | Oncology, hematology, cardiology, neurosurgery |
Symptoms
Individuals with this condition may have symptoms such as seizures due to the compression of the brain tissue or hemorrhaging of angioma scarring surrounding tissue, an intraparenchymal hemorrhage, double vision or other vision problems, language difficulties, memory loss, and incidental hydrocephalus. Minor symptoms may include headaches, weakness or numbness in the arms or legs, and ataxia. When it occurs in the liver it is usually asymptomatic but may present as pain in the upper right abdomen, a feeling of fullness after eating only a small amount of food, lack of an appetite, nausea, and vomiting. In the eye, as the lesion changes in size it will involve the extraocular muscles and optic nerve, and patients report double vision, decreased vision, and progressive proptosis.[3][4]
Presentation
Cavernous hemangiomas can arise nearly anywhere in the body where there are blood vessels. They are often described as raspberry-like structures because of the bubble-like caverns. Unlike the capillary hemangiomas, cavernous ones can be disfiguring and do not tend to regress.
Causes
Most cases of cavernomas are thought to be congenital; however they can develop over the course of a lifetime.[5] While there is no definitive cause, research shows that genetic mutations result in the onset. Congenital hemangiomas that appear on the skin are known as either vascular or red birthmarks.
Familial cerebral cavernous malformations are known to occur. The mutations may be inherited in an autosomal dominant fashion or occur sporadically. Overall familial disease is responsible for one third to one half of cases.[6] In the US approximately 50% of Hispanic patients with cerebral cavernous malformations have a familial form: in contrast in this occurs in only 10 to 20% of Caucasians.[7] The reason for this difference is not presently known.
Several genes – K-Rev interaction trapped 1 (ССМ1), Malcavernin (CCM2) and Programmed cell death protein 10 (ССМ3) – have been identified as having mutations thought to be related to these lesions.[8][9][10] These genes are located at 7q21.2 (chromosome 7 long arm), 7p13 (chromosome 7 short arm)[11] and 3q25.2-q27 (chromosome 3 long arm) respectively. These lesions are further discussed in the Online Mendelian Inheritance in Man site – the reference numbers are OMIM 116860, OMIM 603284 and OMIM 603285 respectively.
Variations
Cerebral cavernomas
Cavernous hemangiomas located in the brain or spinal cord are referred to as cerebral cavernomas or more usually as cerebral cavernous malformations (CCMs),[12][2] and can be found in the white matter, but often abut the cerebral cortex. When they contact the cortex, they can represent a potential seizure focus for the patient. Unlike other cavernous hemangiomas, there is no tissue within the malformation and its borders are not encapsulated. Therefore, they can change in size and number over time.[5]
Liver cavernous hemangioma
Cavernous hemangiomas are the most common benign tumors of the liver.[13] Usually one tumor exists, but multiple lesions can occur in the left or right lobe of the liver in 40% of patients.[3] Their sizes can range from a few millimeters to 20 centimetres. Those over 5 cm are often referred to as giant hemangiomas.[3]
Eye cavernous hemangioma
In the eye, it is known as orbital cavernous hemangioma and is found in women more frequently than men, most commonly between the ages of 20–40.[14] This neoplasm is usually located within the muscle cone, which is lateral to the optic nerve. It is not usually treated unless the patient is symptomatic. Visual impairment happens when the optic nerve is compressed or the extraocular muscles are surrounded.
Mechanism
There are several known causes for cavernous hemangiomas, but some cases are still unknown. Radiation treatment used for other medical conditions has been suggested to cause cavernous malformation in some patients.[15] Hemangioma tumors are a result of rapid proliferation of endothelial cells and pericytic hyperplasia, or the enlargement of tissue as a result of abnormal cell division pericytes. The pathogenesis of hemangioma is still not understood. It has been suggested that growth factors and hormonal influences contribute to the abnormal cell proliferation. Cavernous liver hemangiomas are more commonly diagnosed in women who have been pregnant.[4] As a result of this, it is believed that estrogen levels may play a role in the incidence of liver cavernomas.
Genetic studies show that specific gene mutations or deletions are causes for the disease. The genes identified for cerebral cavernous hemangiomas (or malformations), are CCM1 (also KRIT1), CCM2 (also MGC4607, malcavernin) and CCM3 (also PDCD10). The loss of function of these genes is believed to be responsible for cerebral cavernous malformations.[16] Furthermore, it is also believed that a "second hit mutation" is necessary for the onset of the disease. This means that having a mutation in one of the two genes present on a chromosome is not enough to cause the cavernous malformation, but mutation of both alleles would cause the malformation. Additionally, research on hemangiomas in general has shown that loss of heterozygosity is common in tissue where hemangioma develops.[17] This would confirm that more than a single allele mutation is needed for the abnormal cell proliferation. KRIT1 has been shown to act as a transcription factor in the development of arterial blood vessels in mice. CCM2 has overlapping structure with CCM1 (KRIT1) and acts as a scaffolding protein when expressed. Both genes are involved with MAP3K3 and thus appear to be a part of the same pathway.
CCM2 has been shown to cause embryonic death in mice. Lastly, the CCM3 gene has been shown to have similar expression to CCM1 and CCM2, suggesting a link in its functionality. Currently, no experiments have determined its exact function.[18] The lack of function of these genes in control of a proliferative signaling pathway would result in uncontrolled proliferation and the development of a tumor. In 2018, it was theorized that proliferation of endothelial cells with dysfunctional tight junctions, that are under increased endothelial stress from elevated venous pressure provides the pathophysiological basis for cavernous hemangioma development.[19]
Diagnosis
Gradient-Echo T2WI magnetic resonance imaging (MRI) is most sensitive method for diagnosing cavernous hemangiomas.[20] MRI is such a powerful tool for diagnosis, it has led to an increase in diagnosis of cavernous hemangiomas since the technology's advent in the 1980s.[15] The radiographic appearance is most commonly described as "popcorn" or "mulberry"-shaped.[21] Computed tomography (CT) scanning is not a sensitive or specific method for diagnosing cavernous hemangiomas.[22] Angiography is typically not necessary, unless it is required to rule out other diagnoses. Additionally, biopsies can be obtained from tumor tissue for examination under a microscope. It is essential to diagnose cavernous hemangioma because treatments for these lesions are less aggressive than that of cancerous tumors, such as angiosarcoma. However, since MRI appearance is practically pathognomonic, biopsy is rarely needed for verification.[22]
On ultrasound, cavernous haemangiomas in liver appeared as homogenous, hyperechoic lesions with posterior acoustic enhancement. On CT or MRI scans, it shows peripheral globular/nodular enhancement in the arterial phase, with portions of attenuation of enhancing areas. In the portal venous phase, it shows progressive centripetal enhancement. In delayed phase, it shows retention of contrast. It shows a high signal on T2 weighted images.[23]
Treatment
Asymptomatic lesions may not require treatment but may need to be monitored for any change in the size. A change in size of lesions in the nose, lips, or eyelids can be treated with steroid drugs to slow its progress. Steroids can be taken orally or injected directly into the tumor. Applying pressure to the tumor can also be used to minimize swelling at the site of the hemangioma. A procedure that uses small particles to close off the blood supply is known as sclerotherapy. This allows for tumor shrinkage and less pain. It is possible for the tumor to regrow its blood supply after the procedure has been done.[24] If the lesion caused by the cavernous hemangioma is destroying healthy tissue around it or if the patient is experiencing major symptoms, then surgery can be used to remove the cavernoma piecemeal.[22] A common complication of the surgery is hemorrhage and the loss of blood. There is also the possibility of the hemangioma reoccurring after its removal.[24] Additionally, the risk of a stroke or death is also possible.[25]
Treatments for cerebral cavernous hemangiomas include radiosurgery or microsurgery.[26] The treatment approach depends on the site, size and symptoms present, as well as the history of hemorrhage from the lesion.[26] Microsurgery is generally preferred if the cerebral cavernous hemangioma is superficial in the central nervous system, or the risk of damage to surrounding tissue from irradiation is too high. Additionally, a large hemorrhage with deterioration of the patient or intractable symptoms (such as seizures or coma) are further indications for microsurgical intervention. Gamma-knife radiation is the favored mechanism of radiosurgery. It provides a precise radiation dose to the cerebral cavernous hemangioma while relatively sparing the surrounding tissue.[26] These treatment approaches for cavernous hemangiomas in other regions of the body have limited research.
Prognosis
A few studies have worked on providing details related to the outlook of disease progression. Two studies show that each year 0.5% of people who have never had bleeding from their brain cavernoma, but had symptoms of seizures, were affected by bleeding.[25] In contrast, patients who have had bleeding from their brain cavernoma in the past had a higher risk of being affected by subsequent bleeding. The statistics for this are very broad, ranging from 4–23% a year.[25] Additional studies suggest that women and patients under the age of 40 are at higher risk of bleeding, but similar conducted studies did not reach the same conclusion.[25] However, when cavernous hemangiomas are completely excised, there is very little risk of growth or rebleeding.[27] In terms of life expectancy, not enough data has been collected on patients with this malformation in order to provide a representative statistical analysis.[15]
Epidemiology
The true incidence of cavernous hemangiomas is difficult to estimate because they are frequently misdiagnosed as other venous malformations.[28] Cavernous hemangiomas of the brain and spinal cord (cerebral cavernous hemangiomas (malformations) (CCM)), can appear at all ages but usually occur in the third to fourth decade of a person's life with no sexual preference. In fact, CCM is present in 0.5% of the population. However, approximately 40% of those with malformations have symptoms. Asymptomatic individuals are usually individuals that developed the malformation sporadically, while symptomatic individuals usually have inherited the genetic mutation.[5] The majority of diagnoses of CCM are in adults; however, 25% of cases of CCM are children.[5] Approximately 5% of adults have liver hemangiomas in the United States, but most are asymptomatic.[29] Liver hemangiomas usually occur between the ages of 30–50 and more commonly in women.[4] Cases of infantile liver cavernomas are extremely rare. Cavernous hemangioma of the eye is more prevalent in women than men and between the ages of 20–40.[14]
Research
In the treatment of a brain cavernous hemangioma, neurosurgery is usually the treatment chosen.[30] Research needs to be conducted on the efficacy of treatment with stereotactic radiation therapy, especially on the long-term.[31] However, radiotherapy is still being studied as a form of treatment if neurosurgery is too dangerous due the location of the cavernoma. Genetic researchers are still working on determining the cause of the illness and the mechanism behind blood vessel formation.[25] Clinical trials are being conducted to better assess when it is appropriate to treat a patient with this malformation and with what treatment method.[15] Additionally, long term studies are being conducted because there is no information related to the long-term outlook of patients with cavernoma.[32] An existing registry known as The International Cavernous Angioma Patient Registry[33] collects information from patients diagnosed with cavernoma in order to facilitate discovery of non-invasive treatments.[25]
References
- Awad IA, Polster SP (July 2019). "Cavernous angiomas: deconstructing a neurosurgical disease". Journal of Neurosurgery. 131 (1): 1–13. doi:10.3171/2019.3.JNS181724. PMC 6778695. PMID 31261134.
- Algra A, Rinkel GJ (February 2016). "Prognosis of cerebral cavernomas: on to treatment decisions". The Lancet. Neurology. 15 (2): 129–130. doi:10.1016/S1474-4422(15)00340-3. PMID 26654286.
- Curry MP, Chopra S (2014). "Hepatic Hemangioma". UpToDate. Wolters Kluwer.
- "Liver Hemangioma". MayoClinic. 2013.
- "Cavernous Malformation". Rare Disease Database. National Organization for Rare Disorders, Inc.
- Mindea SA, Yang BP, Shenkar R, Bendok B, Batjer HH, Awad IA (2006) Cerebral cavernous malformations: clinical insights from genetic studies. Neurosurg Focus; 21(1):e1.
- Dashti SR, Hoffer A, Hu YC, Selman WR (July 2006). "Molecular genetics of familial cerebral cavernous malformations". Neurosurgical Focus. 21 (1): e2. doi:10.3171/foc.2006.21.1.3. PMID 16859255.
- Pagenstecher A, Stahl S, Sure U, Felbor U (March 2009). "A two-hit mechanism causes cerebral cavernous malformations: complete inactivation of CCM1, CCM2 or CCM3 in affected endothelial cells". Human Molecular Genetics. 18 (5): 911–8. doi:10.1093/hmg/ddn420. PMC 2640205. PMID 19088124.
- Bergametti F, Denier C, Labauge P, Arnoult M, Boetto S, Clanet M, et al. (January 2005). "Mutations within the programmed cell death 10 gene cause cerebral cavernous malformations". American Journal of Human Genetics. 76 (1): 42–51. doi:10.1086/426952. PMC 1196432. PMID 15543491.
- "Entrez Gene: PDCD10 programmed cell death 10".
- "OMIM Entry * 607929 - CCM2 GENE; CCM2". www.omim.org. Retrieved 2018-05-29.
- "What is a cavernoma?". Cavernoma Alliance UK.
- John TG, Greig JD, Crosbie JL, Miles WF, Garden OJ (December 1994). "Superior staging of liver tumors with laparoscopy and laparoscopic ultrasound". Annals of Surgery. 220 (6): 711–9. doi:10.1097/00000658-199412000-00002. PMC 1234471. PMID 7986136.
- Burkat CN, Pargament J, Yen MT, Goel S, Nerad J. "Cavernous hemangioma". EyeWiki.
- Salman RA. "Symptomatic brain cavernomas" (PDF). Cavernoma Alliance UK.
- Mouchtouris N, Chalouhi N, Chitale A, Starke RM, Tjoumakaris SI, Rosenwasser RH, Jabbour PM (2015). "Management of cerebral cavernous malformations: from diagnosis to treatment". TheScientificWorldJournal. 2015: 808314. doi:10.1155/2015/808314. PMC 4300037. PMID 25629087.
- Marchuk DA (March 2001). "Pathogenesis of hemangioma". The Journal of Clinical Investigation. 107 (6): 665–6. doi:10.1172/jci12470. PMC 208951. PMID 11254664.
- Mattassi R, Loose DA, Vaghi M (2009). Hemangiomas and Vascular Malformations (PDF). Springer-Verlag Italia. ISBN 978-88-470-0568-6. Archived from the original (PDF) on 2014-04-29.
- Maish WN (September 2019). "Developmental venous anomalies and brainstem cavernous malformations: a proposed physiological mechanism for haemorrhage". Neurosurgical Review. 42 (3): 663–670. doi:10.1007/s10143-018-1039-9. PMID 30291476. S2CID 52925057.
- Lehnhardt FG, von Smekal U, Rückriem B, Stenzel W, Neveling M, Heiss WD, Jacobs AH (April 2005). "Value of gradient-echo magnetic resonance imaging in the diagnosis of familial cerebral cavernous malformation". Archives of Neurology. 62 (4): 653–8. doi:10.1001/archneur.62.4.653. PMID 15824268.
- Wang CC, Liu A, Zhang JT, Sun B, Zhao YL (June 2003). "Surgical management of brain-stem cavernous malformations: report of 137 cases". Surgical Neurology. 59 (6): 444–54, discussion 454. doi:10.1016/s0090-3019(03)00187-3. PMID 12826334.
- Greenberg MS (2010-01-01). Handbook of neurosurgery. Greenberg Graphics. ISBN 9781604063264. OCLC 892183792.
- Thampy R, Elsayes KM, Menias CO, Pickhardt PJ, Kang HC, Deshmukh SP, et al. (November 2017). "Imaging features of rare mesenychmal liver tumours: beyond haemangiomas". The British Journal of Radiology. 90 (1079): 20170373. doi:10.1259/bjr.20170373. PMC 5963373. PMID 28766950.
- Miller JM, Morrell N, Quinn RH (August 2018). "Hemangioma". American Academy of Orthopedic Surgeons.
- "Cavernoma". U.K. National Health Service. 5 February 2019.
- Poorthuis MH, Klijn CJ, Algra A, Rinkel GJ, Al-Shahi Salman R (December 2014). "Treatment of cerebral cavernous malformations: a systematic review and meta-regression analysis". Journal of Neurology, Neurosurgery, and Psychiatry. 85 (12): 1319–23. doi:10.1136/jnnp-2013-307349. PMID 24667206.
- Spetzler RF, Yashar K, Peter N. Neurovascular surgery. ISBN 9781604067590. OCLC 967842929.
- "Cavernous malformations". Neurovascular Surgery Brain Aneurysm & AVM Center. Massachusetts General Hospital. Archived from the original on 3 February 2014.
- "Benign Liver Tumors". American Liver Foundation. 2011.
- Poorthuis MH, Klijn CJ, Algra A, Rinkel GJ, Al-Shahi Salman R (December 2014). "Treatment of cerebral cavernous malformations: a systematic review and meta-regression analysis". Journal of Neurology, Neurosurgery, and Psychiatry. 85 (12): 1319–23. doi:10.1136/jnnp-2013-307349. PMID 24667206.
- Poorthuis M, Samarasekera N, Kontoh K, Stuart I, Cope B, Kitchen N, Al-Shahi Salman R (April 2013). "Comparative studies of the diagnosis and treatment of cerebral cavernous malformations in adults: systematic review". Acta Neurochirurgica. 155 (4): 643–9. doi:10.1007/s00701-013-1621-4. PMID 23371401. S2CID 7859921.
- Polster SP, Cao Y, Carroll T, Flemming K, Girard R, Hanley D, et al. (April 2019). "Trial Readiness in Cavernous Angiomas With Symptomatic Hemorrhage (CASH)". Neurosurgery. 84 (4): 954–964. doi:10.1093/neuros/nyy108. PMC 6500884. PMID 29660039.
- "Update Your Cavernous Angioma Patient Registry Profile". Angioma Alliance. Retrieved 2020-08-03.
Further reading
- Robinson JR, Awad IA, Little JR (November 1991). "Natural history of the cavernous angioma". Journal of Neurosurgery. 75 (5): 709–14. doi:10.3171/jns.1991.75.5.0709. PMID 1919692.
- Del Curling O, Kelly DL, Elster AD, Craven TE (November 1991). "An analysis of the natural history of cavernous angiomas". Journal of Neurosurgery. 75 (5): 702–8. doi:10.3171/jns.1991.75.5.0702. PMID 1919691. S2CID 23832599.
| Classification |
|---|