Carroll rearrangement
The Carroll rearrangement is a rearrangement reaction in organic chemistry and involves the transformation of a β-keto allyl ester into a α-allyl-β-ketocarboxylic acid.[1] This organic reaction is accompanied by decarboxylation and the final product is a γ,δ-allylketone. The Carroll rearrangement is an adaptation of the Claisen rearrangement and effectively a decarboxylative allylation.
Reaction mechanism
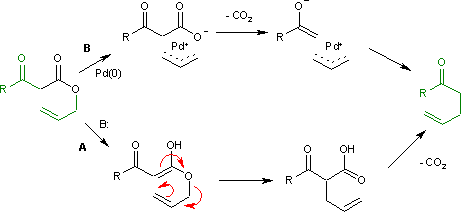
The Carroll rearrangement (1940) in the presence of base and with high reaction temperature (path A) takes place through an intermediate enol which then rearranges in an electrocyclic Claisen rearrangement. The follow-up is a decarboxylation. With palladium(0) as a catalyst, the reaction (Tsuji, 1980) is much milder (path B) with an intermediate allyl cation / carboxylic acid anion organometallic complex.[2]
Decarboxylation precedes allylation as evidenced by this reaction catalyzed by tetrakis(triphenylphosphine)palladium(0):[3]
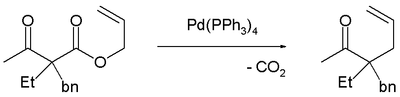
Asymmetric decarboxylative allylation
By introducing suitable chiral ligands, the reaction becomes enantioselective.[4]
The first reported asymmetric rearrangement is catalyzed by tris(dibenzylideneacetone)dipalladium(0) and the Trost ligand:[3]
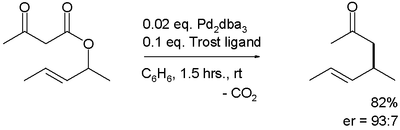
A similar reaction[5] uses additional naphthol.
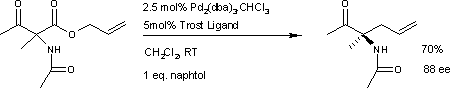
This reaction delivers the main enantiomer with 88% enantiomeric excess. It remains to be seen if this reaction will have a wide scope because the acetamido group appears to be a prerequisite.
The same catalyst but a different ligand is employed in this enantioconvergent reaction:[6]
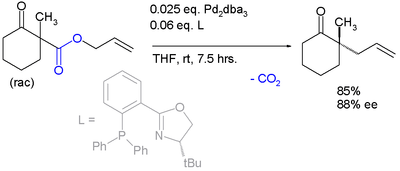
The scope is extended to asymmetric α-alkylation of ketones masked as their enol carbonate esters:[7]
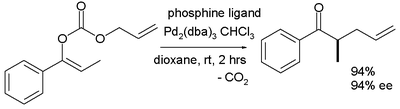
References
- Carroll, M. F. "131. Addition of α,β-unsaturated alcohols to the active methylene group. Part I. The action of ethyl acetoacetate on linalool and geraniol". Journal of the Chemical Society 1940, 704–706. doi:10.1039/JR9400000704
- Palladium-catalyzed rearrangement of allylic esters of acetoacetic acid to give γ,δ-unsaturated methyl ketones Tetrahedron Letters, Volume 21, Issue 33, 1980, Pages 3199-3202 Isao Shimizu, Toshiro Yamada and Jiro Tsuji doi:10.1016/S0040-4039(00)77444-2
- Asymmetric Allylic Alkylation of Ketone Enolates: An Asymmetric Claisen Surrogate Erin C. Burger and Jon A. Tunge Org. Lett.; 2004; 6(22) pp 4113 - 4115; (Letter) doi:10.1021/ol048149t
- Enantioselective Palladium-Catalyzed Decarboxylative Allylic Alkylations Shu-Li You and Li-Xin Dai Angew. Chem. Int. Ed. 2006, 45, 5246 – 5248 doi:10.1002/anie.200601889
- Kuwano, R.; Ishida N.; Murakami, M. "Asymmetric Carroll rearrangement of allyl α-acetamido-β-ketocarboxylates catalysed by a chiral palladium complex". Chemical Communications 2005, (31), 3951–3952. doi:10.1039/b505105c
- Deracemization of Quaternary Stereocenters by Pd-Catalyzed Enantioconvergent Decarboxylative Allylation of Racemic b-Ketoesters Justin T. Mohr, Douglas C. Behenna, Andrew M. Harned, and Brian M. Stoltz Angew. Chem. Int. Ed. 2005, 44, 6924 –6927 doi:10.1002/anie.200502018
- Palladium-Catalyzed Asymmetric Allylic α-Alkylation of Acyclic Ketones Barry M. Trost and Jiayi Xu J. Am. Chem. Soc.; 2005; 127(49) pp 17180 - 17181; (Communication) doi:10.1021/ja055968f