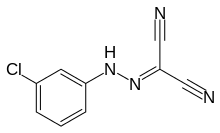
Carbonyl cyanide m-chlorophenyl hydrazone
Carbonyl cyanide m-chlorophenyl hydrazone (CCCP) ([(3-chlorophenyl)hydrazono]malononitrile) is a chemical inhibitor of oxidative phosphorylation. It is a nitrile, hydrazone and protonophore. In general, CCCP causes the gradual destruction of living cells and death of the organism.[1][2] CCCP affects the protein synthesis reactions in seedling mitochondria.[3] CCCP causes an uncoupling of the proton gradient that is established during the normal activity of electron carriers in the electron transport chain. The chemical acts essentially as an ionophore and reduces the ability of ATP synthase to function optimally.
 | |
| Names | |
|---|---|
| IUPAC name
[(3-chlorophenyl)hydrazono]malononitrile | |
| Systematic IUPAC name
[(3-chlorophenyl)hydrazono]propanedinitrile | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.008.277 |
| KEGG | |
| MeSH | CCCP |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C9H5ClN4 | |
| Molar mass | 204.616 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
See also
References
- J.W. Park; S.Y. Lee; J.Y. Yang; H.W. Rho; B.H. Park; S.N. Lim; J.S. Kim; H.R. Kim (1997). "Effect of carbonyl cyanide m-chlorophenylhydrazone (CCmCP) on the dimerization of lipoprotein lipase". Biochimica et Biophysica Acta. 1344 (2): 132–8. doi:10.1016/s0005-2760(96)00146-4. PMID 9030190.
- D. Gášková; B. Brodská; A. Holoubek; K. Sigler (1999). "Factors and processes involved in membrane potential build-up in yeast: diS-C3(3) assay". The International Journal of Biochemistry & Cell Biology. 31 (5): 575–584. doi:10.1016/S1357-2725(99)00002-3. PMID 10399318.
- Y.M. Konstantinov; I.V. Subota; A.S. Arziev. "Protein synthesis in mitochondria under different redox conditions". Preprint of the Irkutsk Institute of Plant Physiology and Biochemistry. Archived from the original on 2011-07-17. Retrieved 2009-02-04.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.