Caffeyl alcohol
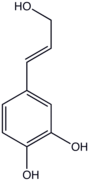
Caffeyl alcohol is the organic compound with the formula (HO)2C6H3-4-CHCHCH2OH. This colourless solid is related to catechol by attachment to allyl alcohol. It is the precursor to one of the three principal lignols.
 | |
| Names | |
|---|---|
| IUPAC name
4-(3-hydroxy-1-propen-1-yl)-1,2-benzenediol, | |
| Other names
Caffeyl alcohol, Caffeoyl alcohol, 3,4-Dihydroxycinnamyl alcohol | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
PubChem CID |
|
| |
| |
| Properties | |
| C9H10O3 | |
| Appearance | White solid |
| Melting point | 144 to 145 °C (291 to 293 °F; 417 to 418 K) |
| moderate | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Preparation and occurrence
In the laboratory, caffeyl alcohol can be synthesized from 3,4-dihydroxybenzaldehyde.[1] It is an intermediate in the biosynthesis of coniferyl alcohol, the conversion being effected by caffeate O-methyltransferase.[2]
Related compounds
Two related compounds are caffeyl aldehyde and caffeic acid, the latter also being a minor component of coffee.[3]
gollark: ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌
gollark: ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌
gollark: ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌
gollark: ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌
gollark: ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌ ❌
References
- Karl Herrmann “Caffeyl Alcohol” Pharmazie 1953, volume 8, 303.
- John M Humphreys, Clint Chapple “Rewriting the Lignin Roadmap” Current Opinion in Plant Biology 2002, volume 5, 224–229. doi:10.1016/S1369-5266(02)00257-1
- Rinantonio Viani, Marino Petracco “Coffee” in Ullmann’s Encyclopedia of Industrial Chemistry” Wiley-VCH, 2007, Weinheim.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.