Cellular Inhibitor of Apoptosis Protein 1
cIAP1 (also named BIRC2) is the abbreviation for a human protein, cellular inhibitor of apoptosis protein-1. It belongs to the IAP family of proteins and therefore contains at least one BIR (baculoviral IAP repeat) domain.[1] cIAP1 is a multi-functional protein which can be found in the cytoplasm of cells and in the nucleus of tumor cells. Its function in this particular case is yet to be understood. However, it is well-known that this protein has a big influence in the growth of diverse cancers. cIAP1 is involved in the development process of osteosarcoma [2] and gastric cancer [3] among others.[4][5]
| cIAP1 | |
|---|---|
| Name | Cellular inhibitor of apoptosis protein 1 |
| Abbreviation | cIAP1 |
| Protein family | Inhibitor of apoptosis proteins |
| Number of amino acids | 618 |
| Molecular weight | 69,9 kDa |
| Human chromosome location | 11 |
Location
The ubication of cIAP1 is diverse depending on the phase of the living cycle of the cell. In healthy cells the protein is usually found in the nucleus. This was experimentally determined by immunofluorescence microscopy and subcellular fractionations methods. However, when the cell is apoptotic nuclear export of cIAP1 is induced provoking an increase in the cytosolic concentration of the protein. When a cell is tumorous it does not cease to proliferate inhibiting the apoptosis, as a result, in cancerous cells cIAP1 is rarely located in the cytoplasm.
In case of dividing cells, cIAP1 is released into the cytosol early in mitosis, then reaccumulated in nucleus in late anaphase and in telophase. Nevertheless, there is a pool of cIAP1 associated to the midbody that acts as the exception to the regular rule.[6]
Structure
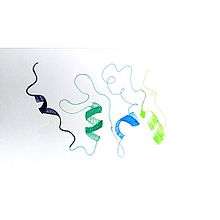
The gene of cIAP1 resides on chromosome 11 and its protein has a quaternary structure. It has a unique protein chain, consequently, is an asymmetric monomer protein. Its tertiary structure is basically composed by alpha domain, formed almost exclusively from alpha helix. Its size is of 31,489 bases composed by 618 amino acids and has a molecular mass of 69900 Da. cIAP1 contains baculovirus IAP repeat domains that facilitate binding to caspases and other proteins. cIAP1 is recruited to TNF receptor complexes where they support cell survival through NF-κB activation while suppressing apoptosis by preventing caspase activation.[7][8][9]
Function
cIAP1 is an inhibitor of apoptosis protein. It directly ubiquitinates RIP1 and induces constitutive RIP1 ubiquitination in cancer cells. Ubiquinated RIP1 associates with the prosurvival kinase TAK1. When this complex is desubiquinated apoptosis is induced.[10][11]
The absence of cIAP1 means that RIP1 will remain nonubiquitinated. As a consequence RIP1 forms a cytosolic complex with the adaptor molecule FADD and caspase 8, which leads to cell death. When cIAP1 ubiquitinates RIP1 this molecule acts as a signal activating the canonical NF-κB signaling pathway. The activation of this pathway stops the noncanonical one and simultaneously the apoptosis.[12]
cIAP1 is important for the activation of MAPK signaling. MAPKs are involved in directing cellular responses to a diverse array os stimuli, such as mitogens, osmotic stress, heat shock and proinflammatory cytokines regulating cell functions including proliferation, gene expression, differentiation, mitosis, cell survival or apoptosis.[13]
cIAP1 is as well implicated in innate immunity. When NOD-like receptors are activated by bacterial peptidoglycans, they oligomerize and recruit cIAP1, cIAP2, TRAF2 and RIP2. This allows cIAP1 mediated ubiquitination of RIP2, which leads to an expression of proinflammatory genes.[14]
Other activities of the cIAP1 have been reported by Yanfei Qi et al. It has got a critical role in controlling β-cell survival under endoplasmatic reticulum (ER) stress. Studies show that when the protein is exposed to palmitate the concentration of cIAP1 decreases and, as a result, the apoptosis is no longer inhibited resulting in the death of the cell. ER stress increases cIAP1 expression in cancer cells through the UPR pathway, that is why, the induction of cIAP1 is suggested to be important for cancerous cell survival under stress conditions.[15]
Related diseases
Crohn's disease. cIAP1 is responsible for NOD signalling. When this signalling is defective, Crohn's disease is triggered. The most general symptoms of the disease are diarrhea, rectal bleeding and abdominal cramps and pain among others.[16][17]
Pancreatic, liver, lung and oseophageal cancer. cIAP1 overexpression is directly related to the proliferation of the previously mentioned types of cancer. Several courses of treatment are focused on the removal of the IAPs to induce cells cytotoxicity.[18]
Hemorrhage and Vascular Regression. cIAP1 has an important role on the maintenance of endothelial cells and blood vessel homeostasis during the development of the vessels. Mutations on the gene that encodes cIAP1 are related to hemorrhage and vascular regression because of the defects it represents on the endothelial cell survival and the modification of apoptosis.[19]
References
- Eckelman, B. P.; Drag, M.; Snipas, S. J.; Salvesen, G. S. (2008-02-01). "The mechanism of peptide-binding specificity of IAP BIR domains". Cell Death & Differentiation. 15 (5): 920–928. doi:10.1038/cdd.2008.6. ISSN 1350-9047. PMID 18239672.
- Ma, Ou; Cai, Wei-Wen; Zender, Lars; et al. (2009). "MMP13, Birc2 (cIAP1) and Birc3 (cIAP2), Amplified on Chromosome 9A1, Collaborate with p53 Deficiency in Mouse Osteosarcoma Progression". Cancer Res. 69 (6): 2559–67. doi:10.1158/0008-5472.CAN-08-2929. PMC 2663905. PMID 19276372.
- Park YM, Hong SW, Moon JH, Shin JS, Lee HR, Ha SH, Lee DH, Kim JH, Kim SM, Kim JE, Kim KP, Hong YS, Choi EK, Lee JS, Jin DH, Kim T, Jung SA (2015). "Cellular inhibitor of apoptosis protein 1 (cIAP1) stability contributes to YM155 resistance in human gastric cancer cells". J Biol Chem. 290 (16): 9974–85. doi:10.1074/jbc.M114.600874. PMC 4400372. PMID 25635055.
- Wang H, Zhang B, Chen Y, Zhang Y, Sun X, Xiao G, Nan K, Ren H, Qin S, Yang C (2016). "LCL161 increases paclitaxel-induced apoptosis by degrading cIAP1 and cIAP2 in NSCLC". J Exp Clin Cancer Res. 35 (1): 158. doi:10.1186/s13046-016-0435-7. PMC 5062899. PMID 27737687.
- Jean Berthelet; Arthur Marivin; Simon Gemble; Valérie Edmond; Stéphanie Plenchette; Brice Lagrange; Arlette Hammann; Alban Dupoux; Laurent Delva; Béatrice Eymin; Eric Solary; Laurence Dubrez; Jessy Cartier (2011). "Cellular Inhibitor of Apoptosis Protein-1 (cIAP1) Can Regulate E2F1 Transcription Factor-mediated Control of Cyclin Transcription". J Biol Chem. 286 (30): 26406–17. doi:10.1074/jbc.M110.191239. PMC 3143604. PMID 21653699.
- Okada K, Hyer M, Welsh K, Zapata JM, Reed JC, Samuel T (2005). "cIAP1 Localizes to the nuclear compartment and modulates the cell cycle". Cancer Res. 65 (1): 210–8. PMID 15665297.
- Mahoney, D.J. (2008). "Both cIAP1 and cIAP2 regulate TNF-mediated NF-B activation" (PDF). Proceedings of the National Academy of Sciences. 105 (33): 11778–11783. doi:10.1073/pnas.0711122105. PMC 2575330. PMID 18697935. Retrieved October 23, 2016.
- "BIRC2 Gene(Protein Coding) Baculoviral IAP Repeat Containing 2". GeneCards. Retrieved October 23, 2016.
- Welsh, Kate (2016). "Characterization of Potent SMAC Mimetics that Sensitize Cancer Cells to TNF Family-Induced Apoptosis". PLOS One. 11 (9): e0161952. doi:10.1371/journal.pone.0161952. PMC 5019375. PMID 27617834.
- Milutinovic S, Dickson KM, Ho WC, Boudreault A, Durkin J, Gillard JW, Jaquith JB, Morris SJ, Barker PA, Bertrand MJ (June 2008). "cIAP1 and cIAP2 facilitate cancer cell survival by functioning as E3 ligases that promote RIP1 ubiquitination". Mol. Cell. 30 (6): 689–700. doi:10.1016/j.molcel.2008.05.014. PMID 18570872.
- "UniProtKB - Q13490 (BIRC2_HUMAN)". Uniprot.
- Holcik M, Graber TE (2011). "Distinct roles for the cellular inhibitors of apoptosis proteins 1 and 2". Cell Death & Disease. 2 (3): e135. doi:10.1038/cddis.2011.20. PMC 3101816. PMID 21430708.
- Robinson F, Beers Gibson T, Xu BE, Karandikar M, Berman K, Cobb MH, Pearson G (April 2001). "Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions". Endocr. Rev. 22 (2): 153–83. doi:10.1210/er.22.2.153. PMID 11294822.
- Vucic, D.; Almagro, M.C. (2012). "The inhibitor of apoptosis (IAP) proteins are critical regulators of signaling pathways and targets for anti-cancer therapy". Experimental Oncology. 34 (3): 200–11. PMID 23070005.
- Pu Xia; Yanfei Qi (2012). "Cellular Inhibitor of Apoptosis Protein-1 (cIAP1) Plays a Critical Role in β-Cell Survival under Endoplasmic Reticulum Stress". J Biol Chem. 287 (38): 32236–45. doi:10.1074/jbc.M112.362160. PMC 3442554. PMID 22815481.
- Doiron K, Labbé K, Korneluk RG, Barker PA, Saleh M, Bertrand MJ (2009). "Cellular inhibitors of apoptosis cIAP1 and cIAP2 are required for innate immunity signaling by the pattern recognition receptors NOD1 and NOD2". Immunity. 30 (6): 789–801. doi:10.1016/j.immuni.2009.04.011. PMID 19464198.
- "Crohn's Disease is a Chronic Condition Crohn's Disease is a Chronic Condition By understanding your body and managing your symptoms, you can live a full and rewarding life What is Crohn's Disease?".
- David Vaux; Gerry Melino. Cell Death. Wiley.
- Temesgen Samuel; Tracy Mitchell; John C. Reed; Didier Y. R. Stainier; Massimo M. Santoro. "Birc2 (cIap1) regulates endothelial cell integrity and blood vessel homeostasis".