C1QBP
Complement component 1 Q subcomponent-binding protein, mitochondrial is a protein that in humans is encoded by the C1QBP gene.[5][6][7]
The human complement subcomponent C1q associates with C1r and C1s in order to yield the first component of the serum complement system. The protein encoded by this gene is known to bind to the globular heads of C1q molecules and inhibit C1 activation. This protein has also been identified as the p32 subunit of pre-mRNA splicing factor SF2, as well as a hyaluronic acid-binding protein.[7]
Protein subunit
C1QBP is 282 amino acid in length and has three homologous subunit with its N-terminal 73 amino acid residues cleaved off to produce mature C1QBP. C1QBP appears as a monomer around 33 kDa on SDS-PAGE gel under both reducing and nonreducing condition but migrates as a trimer on size-exclusion chromatography (gel filtration).[8]
Protein structure
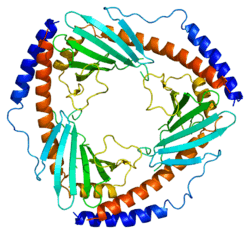
The crystal structure of C1QBP at 2.25 Å resolution shows a homotrimeric ring displaying symmetry. The individual subunits are held together by noncovalent interactions and forms a doughnut shaped quaternary structure with a central cavity of 20 Å in diameter. Each Subunit of C1QBP has seven β-strand (β1- β7) and three α-helices (α1- α3). C1QBP is negatively charged on its soluble face while the membrane face is predominantly positively charged.[9]
Interactions
C1QBP has been shown to interact with Protein kinase D1,[10] BAT2,[11] PRKCD,[10] PKC alpha[10] and Protein kinase Mζ.[10] Other interacting partners of C1QBP include protein domains from pathogens such as bacteria,[12] virus [13] and plasmodium falciparum.[14] Plasma proteins including fibrinogen, FXII and HK have been demonstrated to interact with C1QBP in a zinc dependent manner,.[15][16] Recently, a tumour homing peptide, LyP-1(CGNKRTRGC) has been shown to selectively bind to C1QBP in tumour expressing cells.[17]
References
- GRCh38: Ensembl release 89: ENSG00000108561 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000018446 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- Deb TB, Datta K (March 1996). "Molecular cloning of human fibroblast hyaluronic acid-binding protein confirms its identity with P-32, a protein co-purified with splicing factor SF2. Hyaluronic acid-binding protein as P-32 protein, co-purified with splicing factor SF2". J Biol Chem. 271 (4): 2206–12. doi:10.1074/jbc.271.4.2206. PMID 8567680.
- Ghebrehiwet B, Lim BL, Peerschke EI, Willis AC, Reid KB (June 1994). "Isolation, cDNA cloning, and overexpression of a 33-kD cell surface glycoprotein that binds to the globular "heads" of C1q". J Exp Med. 179 (6): 1809–21. doi:10.1084/jem.179.6.1809. PMC 2191527. PMID 8195709.
- "Entrez Gene: C1QBP complement component 1, q subcomponent binding protein".
- Jiang, Jianzhong (1999). "rystal structure of human p32, a doughnut-shaped acidic mitochondrial matrix protein". PNAS. 96 (7): 3572–3577. Bibcode:1999PNAS...96.3572J. doi:10.1073/pnas.96.7.3572. PMC 22335. PMID 10097078.
- Jiang, Jianzhong (1999). "rystal structure of human p32, a doughnut-shaped acidic mitochondrial matrix protein". PNAS. 96 (7): 3572–3577. Bibcode:1999PNAS...96.3572J. doi:10.1073/pnas.96.7.3572. PMC 22335. PMID 10097078.
- Storz, P; Hausser A; Link G; Dedio J; Ghebrehiwet B; Pfizenmaier K; Johannes F J (August 2000). "Protein kinase C [micro] is regulated by the multifunctional chaperon protein p32". J. Biol. Chem. UNITED STATES. 275 (32): 24601–7. doi:10.1074/jbc.M002964200. ISSN 0021-9258. PMID 10831594.
- Lehner, Ben; Semple Jennifer I; Brown Stephanie E; Counsell Damian; Campbell R Duncan; Sanderson Christopher M (January 2004). "Analysis of a high-throughput yeast two-hybrid system and its use to predict the function of intracellular proteins encoded within the human MHC class III region". Genomics. United States. 83 (1): 153–67. doi:10.1016/S0888-7543(03)00235-0. ISSN 0888-7543. PMID 14667819.
- Braun, Laurence; Ghebrehiwet, Berhane; Cossart, Pascale (2000). "gC1q-R/p32, a C1q-binding protein, is a receptor for the InlB invasion protein of Listeria monocytogenes". The EMBO Journal. 19 (7): 1458–1466. doi:10.1093/emboj/19.7.1458. PMC 310215. PMID 10747014.
- Kittlesen, David J.; Chianese-Bullock, Kimberly A.; Yao, Zhi Q; Braciale, Thomas J.; Hahn, Young S. (2000). "Interaction between complement receptor gC1qR and hepatitis C virus core protein inhibits T-lymphocyte proliferation". J Clin Invest. 106 (10): 1239–1249. doi:10.1172/jci10323. PMC 381434. PMID 11086025.
- Magallón-Tejada, Ariel (2016). "Cytoadhesion to gC1qR through Plasmodium falciparum Erythrocyte Membrane Protein 1 in Severe Malaria". PLoS Pathog. 12 (11): e1006011. doi:10.1371/journal.ppat.1006011. PMC 5106025. PMID 27835682.
- Pathak, Monika; Kaira, Bubacarr G.; Slater, Alexandre; Emsley, Jonas (2018). "Cell Receptor and Cofactor Interactions of the Contact Activation System and Factor XI". Frontiers in Medicine. 5: 66. doi:10.3389/fmed.2018.00066. PMC 5871670. PMID 29619369.
- Peerschke, EI; Bayer, AS; Ghebrehiwet, B; Xiong, YQ (2006). "gC1qR/p33 blockade reduces Staphylococcus aureus colonization of target tissues in an animal model of infective endocarditis". Infect Immun. 74 (8): 4418–4423. doi:10.1128/iai.01794-05. PMC 1539591. PMID 16861627.
- Paasonen, Lauri; Sharma, Shweta; Braun, Gary B.; Kotamraju, Venkata R.; Chung, Thomas D.Y.; She, Zhigang; Sugahara, Kazuki N; Yliperttula, Marjo; Wu, Bainan; Pellecchia, Maurizio; Ruoslahti, Erkki; Teesalu, Tambet (2017). "New p32/gC1qR Ligands for Targeted Tumor Drug Delivery". ChemBioChem. 17 (7): 570–575. doi:10.1002/cbic.201500564. PMC 5433940. PMID 26895508.
Further reading
- Krainer AR, Mayeda A, Kozak D, Binns G (1991). "Functional expression of cloned human splicing factor SF2: homology to RNA-binding proteins, U1 70K, and Drosophila splicing regulators". Cell. 66 (2): 383–94. doi:10.1016/0092-8674(91)90627-B. PMID 1830244.
- Busby TF, Ingham KC (1990). "NH2-terminal calcium-binding domain of human complement C1s- mediates the interaction of C1r- with C1q". Biochemistry. 29 (19): 4613–8. doi:10.1021/bi00471a016. PMID 2372546.
- Fridell RA, Harding LS, Bogerd HP, Cullen BR (1995). "Identification of a novel human zinc finger protein that specifically interacts with the activation domain of lentiviral Tat proteins". Virology. 209 (2): 347–57. doi:10.1006/viro.1995.1266. PMID 7778269.
- Luo Y, Yu H, Peterlin BM (1994). "Cellular protein modulates effects of human immunodeficiency virus type 1 Rev". J. Virol. 68 (6): 3850–6. PMC 236890. PMID 8189522.
- Honoré B, Madsen P, Rasmussen HH, et al. (1994). "Cloning and expression of a cDNA covering the complete coding region of the P32 subunit of human pre-mRNA splicing factor SF2". Gene. 134 (2): 283–7. doi:10.1016/0378-1119(93)90108-F. PMID 8262387.
- Tange TO, Jensen TH, Kjems J (1996). "In vitro interaction between human immunodeficiency virus type 1 Rev protein and splicing factor ASF/SF2-associated protein, p32". J. Biol. Chem. 271 (17): 10066–72. doi:10.1074/jbc.271.17.10066. PMID 8626563.
- Guo N, Weremowicz S, Lynch N, et al. (1997). "Assignment of C1QBP encoding the C1q globular domain binding protein (gC1q-R) to human chromosome 17 band p13.3 by in situ hybridization". Cytogenet. Cell Genet. 77 (3–4): 283–4. doi:10.1159/000134598. PMID 9284938.
- Majumdar M, Datta K (1998). "Assignment of cDNA encoding hyaluronic acid-binding protein 1 to human chromosome 17p12-p13". Genomics. 51 (3): 476–7. doi:10.1006/geno.1998.5364. PMID 9721222.
- Petersen-Mahrt SK, Estmer C, Ohrmalm C, et al. (1999). "The splicing factor-associated protein, p32, regulates RNA splicing by inhibiting ASF/SF2 RNA binding and phosphorylation". EMBO J. 18 (4): 1014–24. doi:10.1093/emboj/18.4.1014. PMC 1171193. PMID 10022843.
- Jiang J, Zhang Y, Krainer AR, Xu RM (1999). "Crystal structure of human p32, a doughnut-shaped acidic mitochondrial matrix protein". Proc. Natl. Acad. Sci. U.S.A. 96 (7): 3572–7. Bibcode:1999PNAS...96.3572J. doi:10.1073/pnas.96.7.3572. PMC 22335. PMID 10097078.
- Braun L, Ghebrehiwet B, Cossart P (2000). "gC1q-R/p32, a C1q-binding protein, is a receptor for the InlB invasion protein of Listeria monocytogenes". EMBO J. 19 (7): 1458–66. doi:10.1093/emboj/19.7.1458. PMC 310215. PMID 10747014.
- Beatch MD, Hobman TC (2000). "Rubella virus capsid associates with host cell protein p32 and localizes to mitochondria". J. Virol. 74 (12): 5569–76. doi:10.1128/JVI.74.12.5569-5576.2000. PMC 112044. PMID 10823864.
- Storz P, Hausser A, Link G, et al. (2000). "Protein kinase C [micro] is regulated by the multifunctional chaperon protein p32". J. Biol. Chem. 275 (32): 24601–7. doi:10.1074/jbc.M002964200. PMID 10831594.
- Tye AJ, Ghebrehiwet B, Guo N, et al. (2001). "The human gC1qR/p32 gene, C1qBP. Genomic organization and promoter analysis". J. Biol. Chem. 276 (20): 17069–75. doi:10.1074/jbc.M009064200. PMID 11278463.
- Schaerer MT, Kannenberg K, Hunziker P, et al. (2001). "Interaction between GABA(A) receptor beta subunits and the multifunctional protein gC1q-R". J. Biol. Chem. 276 (28): 26597–604. doi:10.1074/jbc.M102534200. PMID 11350968.
- Kobayashi M, Hanai R (2001). "M phase-specific association of human topoisomerase IIIbeta with chromosomes". Biochem. Biophys. Res. Commun. 287 (1): 282–7. doi:10.1006/bbrc.2001.5580. PMID 11549288.
- Rozanov DV, Ghebrehiwet B, Postnova TI, et al. (2002). "The hemopexin-like C-terminal domain of membrane type 1 matrix metalloproteinase regulates proteolysis of a multifunctional protein, gC1qR". J. Biol. Chem. 277 (11): 9318–25. doi:10.1074/jbc.M110711200. PMID 11773076.
- Majumdar M, Meenakshi J, Goswami SK, Datta K (2002). "Hyaluronan binding protein 1 (HABP1)/C1QBP/p32 is an endogenous substrate for MAP kinase and is translocated to the nucleus upon mitogenic stimulation". Biochem. Biophys. Res. Commun. 291 (4): 829–37. doi:10.1006/bbrc.2002.6491. PMID 11866440.