Brivanib alaninate
Brivanib alaninate (INN/USAN) also known as BMS-582664 is an investigational, anti-tumorigenic drug for oral administration. The drug is being developed by Bristol-Myers Squibb for the treatment of hepatocellular carcinoma or HCC (also called malignant hepatoma), the most common type of liver cancer.
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.167.334 |
| Chemical and physical data | |
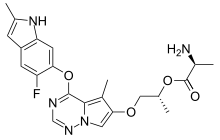
| Formula | C22H24FN5O4 |
| Molar mass | 441.463 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Brivanib alaninate is a multitargeted tyrosine kinase inhibitor (as is sorafenib).
Brivanib alaninate also inhibits VEGFR and fibroblast growth factor receptors (FGFR), which is known to play a major role in the etiopathogenesis of HCC. To date, brivanib alaninate has been investigated in 29 studies, including more than 4,000 patients around the world.
Hepatocellular carcinoma (summary)
Hepatocellular carcinoma [1] is a primary cancer of the liver and is more common in men than in women. The disease occurs mostly in people who have scarring of the liver (cirrhosis) or after infection with hepatitis B or hepatitis C. Symptoms include pain and swelling in the abdomen, weight loss, weakness, loss of appetite, and nausea. Hepatocellular carcinoma is a severe and life-threatening disease that is associated with poor overall survival.[2] While the choice of treatment depends mainly on how advanced the disease is, the only proven therapies to cure the cancer are either surgical removal of the tumors or remove and replace the liver via transplantation, but these therapies can only be carried out in very few patients. Other treatments include chemotherapy and immunotherapy. Radiofrequency ablation and ethanol injection are also used to remove small tumors.[3]
As a result of poor liver function, metastases, or both, only 10% to 20% of patients undergo surgery. In patients having surgery, the 5-year survival rate is only 25% to 50%. Several chemotherapeutic agents have been evaluated for the treatment of hepatocellular carcinoma. Doxorubicin (trade name Adriamycin; also known as hydroxydaunorubicin), the most widely used agent in HCC, has shown a 4% to 10.5% response rate in patients with HCC. Studies have shown that the overall response (OR) rate, but not overall survival (OS), doubles when doxorubicin was given in combination with cisplatin, IFN, and 5-fluorouracil. The multi-targeted tyrosine kinase inhibitor sorafenib (trade name Nexavar), which inhibits vascular endothelial growth factor receptor (VEGFR), platelet-derived growth factor receptor, raf, c-kit, and flt-3, has been shown to inhibit HCC-induced proliferation and angiogenesis. Sorafenib has also been shown to provide a significant improvement in OS in patients with HCC. Based on these results, researchers concluded that this class of agents may be effective in the treatment of HCC.
Biological activity
Brivanib is the alanine ester of a VEGFR-2 inhibitor BMS-540215 and is hydrolyzed to the active moiety BMS-540215 in vivo. BMS-540215, a dual tyrosine kinase inhibitor, shows potent and selective inhibition of VEGFR and fibroblast growth factor receptor (FGFR) tyrosine kinases.[4][5]
BMS-540215 is an ATP-competitive inhibitor of human VEGFR-2, with an IC50 of 25 nmol/L and Ki of 26 nmol/L. In addition, it inhibits VEGFR-1 (IC50 = 380 nmol/L) and VEGFR-3 (IC50 = 10 nmol/L). BMS-540215 also showed good selectivity for FGFR-1 (IC50 = 148 nmol/L), FGFR-2 (IC50 = 125 nmol/L), and FGFR-3 (IC50 = 68 nmol/L). Furthermore, BMS-540215 has been shown to selectively inhibit the proliferation of endothelial cells stimulated by VEGF and FGF in vitro with IC50 values of 40 and 276 nmol/L, respectively.[6][7] It also shows broad-spectrum in vivo antitumor activity over multiple dose levels and induces stasis in large tumors, suggesting that it may have a role in the treatment of hepatocellular carcinoma (HCC).
Pharmacokinetic and pharmacodynamic profiles
BMS-582664 was originally prepared in an effort to improve the aqueous solubility and oral bioavailability of the parent compound BMS-540215. Both BMS-540215 and its orally active ester prodrug BMS-582664 (brivanib alaninate), demonstrate broad-spectrum in vivo antitumor activity over multiple dose levels as well as being effective at inducing stasis in large tumors. Brivanib alaninate can also be safely dosed in combination with other targeted and cytotoxic drugs including paclitaxel resulting in enhanced antitumor activity as observed by a long period of tumor growth inhibition. Overall survival was significantly extended by brivanib versus sorafenib, both first- line and when second.
Mechanisms of action
The exact mechanisms by which brivanib treatment induces growth inhibition are not well understood. Ongoing research has shown that brivanib affects the host endothelium based on both in vitro and in vivo effects). Brivanib may prevent the tumor mass from expanding by cutting off the supply of nutrients and growth factors to the tumor cells.
A recent study showed that brivanib effectively inhibits tumor growth and that brivanib-induced growth inhibition is associated with inactivation of VEGFR-2, increased apoptosis, a reduction in microvessel density, inhibition of cell proliferation, and down-regulation of cell cycle regulators, including cyclin D1, Cdk-2, Cdk-4, cyclin B1, and phospho-c-Myc.[4] Based on this study, researchers have concluded that cell cycle arrest due to a reduction in positive cell cycle regulators may be responsible for the observed growth inhibition. The same study showed that treatment with brivanib also led to a decrease in the number of proliferating cells compared with control.
Dosing
Doses required for achieving complete tumor stasis do not produce overt toxicity as defined by weight loss, mortality or unkempt appearance and behavior. The prodrug brivanib alaninate, which is completely hydrolyzed to BMS-540215 in vivo, has pharmacokinetic properties suitable for once a day or twice daily oral dosing. Completed and ongoing clinical trials show that brivanib alaninate appears to be tolerable and may exhibits favorable pharmacokinetic and pharmacodynamic profiles with evidence of target inhibition in surrogate tissues. Clinical and pharmacodynamic data support an oral once daily administration at 800 mg. The investigational drug shows promising activity as single agent in HCC. Brivanib also shows promising activity in combination with cetuximab in colorectal cancer.[8] Further evaluations are currently ongoing.
Safety profile
Ongoing clinical trials have shown brivanib to have a manageable safety profile. The trial drug is one of the first agents to demonstrate promising antitumor activity in advanced HCC patients treated with prior sorafenib.[9]
Adverse reactions
In clinical trials, brivanib was generally well tolerated. The most common adverse events included fatigue, hypertension, and diarrhea.[10]
Ongoing clinical development
While a phase II trial for hepatocellular carcinoma showed an acceptable safety profile and results indicating efficacy against HCC, four subsequent phase III trials found no increased survival and increased rates of adverse effects when compared with sorafenib or placebo.[11]
Regulatory status
On 27 October 2011, orphan designation (EU/3/11/918) was granted by the European Commission to Bristol-Myers Squibb for brivanib alaninate for the treatment of hepatocellular carcinoma.[12] At the time of the orphan designation, several medicines were authorized in the EU for the treatment of hepatocellular carcinoma.
Submission and application
At the time of submission of the application for orphan designation, clinical trials with brivanib alaninate in patients with hepatocellular carcinoma were ongoing. As part of the submission process, Bristol-Myers Squibb has provided sufficient information to show that brivanib alaninate might be of significant benefit for patients with hepatocellular carcinoma because it could provide an alternative for patients who cannot take or for whom existing treatments do not work. Early studies show that it might improve the treatment of patients with this condition, particularly if used when existing treatment had failed. However, this assumption needs to be confirmed at the time of EU marketing authorization, in order to maintain the orphan status.
References
- National Cancer Institute Dictionary of Cancer Terms
- National Cancer Institute Adult Primary Liver Cancer Treatment (PDQ®)
- National Cancer Institute Adult Primary Liver Cancer Treatment (PDQ®)/Treatment Option Overview
- Huynh H, Ngo VC, Fargnoli J, Ayers M, Soo KC, Koong HN, et al. (October 2008). "Brivanib alaninate, a dual inhibitor of vascular endothelial growth factor receptor and fibroblast growth factor receptor tyrosine kinases, induces growth inhibition in mouse models of human hepatocellular carcinoma". Clinical Cancer Research. 14 (19): 6146–53. doi:10.1158/1078-0432.CCR-08-0509. PMID 18829493.
- Cai ZW, Zhang Y, Borzilleri RM, Qian L, Barbosa S, Wei D, et al. (March 2008). "Discovery of brivanib alaninate ((S)-((R)-1-(4-(4-fluoro-2-methyl-1H-indol-5-yloxy)-5-methylpyrrolo[2,1-f][1,2,4]triazin-6-yloxy)propan-2-yl)2-aminopropanoate), a novel prodrug of dual vascular endothelial growth factor receptor-2 and fibroblast growth factor receptor-1 kinase inhibitor (BMS-540215)". Journal of Medicinal Chemistry. 51 (6): 1976–80. doi:10.1021/jm7013309. PMID 18288793.
- Ayers M, Fargnoli J, Lewin A, Wu Q, Platero JS (July 2007). "Discovery and validation of biomarkers that respond to treatment with brivanib alaninate, a small-molecule VEGFR-2/FGFR-1 antagonist". Cancer Research. 67 (14): 6899–906. doi:10.1158/0008-5472.CAN-06-4555. PMID 17638901.
- Bhide RS, Cai ZW, Zhang YZ, Qian L, Wei D, Barbosa S, et al. (April 2006). "Discovery and preclinical studies of (R)-1-(4-(4-fluoro-2-methyl-1H-indol-5-yloxy)-5- methylpyrrolo[2,1-f][1,2,4]triazin-6-yloxy)propan- 2-ol (BMS-540215), an in vivo active potent VEGFR-2 inhibitor". Journal of Medicinal Chemistry. 49 (7): 2143–6. doi:10.1021/jm051106d. PMID 16570908.
- Clinical trial number NCT00640471 for "Cetuximab With or Without Brivanib in Treating Patients With K-Ras Wild Type Tumours and Metastatic Colorectal Cancer" at ClinicalTrials.gov
- Allen E, Walters IB, Hanahan D (August 2011). "Brivanib, a dual FGF/VEGF inhibitor, is active both first and second line against mouse pancreatic neuroendocrine tumors developing adaptive/evasive resistance to VEGF inhibition". Clinical Cancer Research. 17 (16): 5299–310. doi:10.1158/1078-0432.CCR-10-2847. PMC 3156934. PMID 21622725.
- Finn RS, Kang YK, Mulcahy M, Polite BN, Lim HY, Walters I, et al. (April 2012). "Phase II, open-label study of brivanib as second-line therapy in patients with advanced hepatocellular carcinoma". Clinical Cancer Research. 18 (7): 2090–8. doi:10.1158/1078-0432.CCR-11-1991. PMID 22238246.
- "CASE STUDIES WHERE PHASE 2 AND PHASE 3 TRIALS HAD DIVERGENT RESULTS" (PDF). www.fda.gov. 2017. Retrieved 2019-06-06.
- orphan designation
External links
- Clinical trial number NCT00798252 for "Ascending Multiple-Dose Study of Brivanib Alaninate in Combination With Chemotherapeutic Agents in Subjects With Advanced Cancers" at ClinicalTrials.gov
- Clinical trial number NCT00437437 for "A Phase I Study to Determine the Effect of Food on Brivanib (BMS-582664)" at ClinicalTrials.gov
- Clinical trial number NCT00633789 for "Phase II Study of Brivanib (BMS-582664) to Treat Multiple Tumor Types" at ClinicalTrials.gov
- Clinical trial number NCT00888173 for "Brivanib Alaninate in Treating Patients With Recurrent or Persistent Endometrial Cancer" at ClinicalTrials.gov
- Clinical trial number NCT01253668 for "Brivanib Metastatic Renal Cell Carcinoma" at ClinicalTrials.gov
- Public summary of opinion on orphan designation. Brivanib alaninate for the treatment of hepatocellular carcinoma
- New drug information/Abbreviated Scientific Narrative