Borophene
Borophene is a crystalline atomic monolayer of boron, i.e., it is a two-dimensional allotrope of boron and also known as boron sheet. First predicted by theory in the mid-1990s,[1] different borophene structures were experimentally confirmed in 2015.[2][3]
Properties
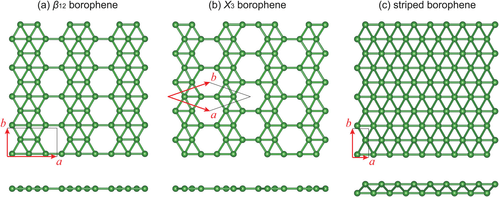
Experimentally various atomically-thin, crystalline and metallic borophenes were synthesized on clean metal surfaces under ultrahigh-vacuum conditions.[2][3] Their atomic structure consists of mixed triangular and hexagonal motifs, such as shown in Figure 1. The atomic structure is a consequence of an interplay between two-center and multi-center in-plane bonding, which is typical for electron deficient elements like boron.[4]
Borophenes exhibit in-plane elasticity and ideal strength. They can be stronger than graphene, and more flexible, in some configurations.[5] For example, boron nanotubes have a higher 2D Young's modulus than any other known carbon and noncarbon nanostructures.[6] Borophenes undergo novel structural phase transition under in-plane tensile loading due to the fluxional nature of their multi-center in-plane bonding.[7] Borophene has potential as an anode material for batteries due to high theoretical specific capacities, electronic conductivity and ion transport properties. Hydrogen easily adsorbs to borophene, offers potential for hydrogen storage – over 15% of its weight. Borophene can catalyze the breakdown of molecular hydrogen into hydrogen ions, and reduce water.[5]
History
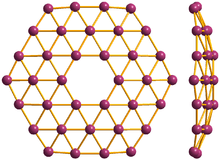
36 cluster might be seen as smallest borophene; front and side view
Computational studies by I. Boustani and A. Quandt showed that small boron clusters do not adopt icosahedral geometries like boranes, instead they turn out to be quasi-planar (see Figure 2).[1] This led to the discovery of a so-called Aufbau principle[8] that predicts the possibility of borophene (boron sheets),[1] boron fullerenes (borospherene)[9] and boron nanotubes.[10][11][12]
Additional studies showed that extended, triangular borophene (Figure 1(c)) is metallic and adopts a non-planar, buckled geometry.[13][14] Further computational studies, initiated by the prediction of a stable B80 boron fullerene,[15] suggested that extended borophene sheets with honeycomb structure and with partially filled hexagonal holes are stable.[16][17] These borophene structures were predicted to be metallic. The so-called γ sheet (a.k.a. β12 borophene or υ1/6 sheet) is shown in Figure 1(a).[17]
The planarity of boron clusters was first experimentally confirmed by the research team of L.-S. Wang.[18] Later they showed that the structure of B
36 (see Figure 2) is the smallest boron cluster to have sixfold symmetry and a perfect hexagonal vacancy, and that it can serve as a potential basis for extended two-dimensional boron sheets.[19]
After the synthesis of silicene, multiple groups predicted that borophene could potentially be realized with the support of a metal surface.[20][21][22] In particular, the lattice structure of borophene was shown to depend on the metal surface, displaying a disconnect from that in a freestanding state.[23]
In 2015 two research teams succeeded in synthesizing different borophene phases on silver (111) surfaces under ultrahigh-vacuum conditions.[2][3] Among the three borophene phases synthesized (see Figure 1), the v1/6 sheet, or β12, was shown by an earlier theory to be the ground state on the Ag(111) surface,[23] while the χ3 borophene was previously predicted by Zeng team in 2012.[24] So far, borophenes exist only on substrates; how to transfer them onto a device-compatible substrate is necessary, but remains a challenge.[25]
Atomic-scale characterization, supported by theoretical calculations, revealed structures reminiscent of fused boron clusters consisting of mixed triangular and hexagonal motives, as previously predicted by theory and shown in Figure 1. Scanning tunneling spectroscopy confirmed that the borophenes are metallic. This is in contrast to bulk boron allotropes, which are semiconducting and marked by an atomic structure based on B12 icosahedra.
See also
References
- Boustani, Ihsan (January 1997). "New quasi-planar surfaces of bare boron". Surface Science. 370 (2–3): 355–363. Bibcode:1997SurSc.370..355B. doi:10.1016/S0039-6028(96)00969-7.
- Mannix, A. J.; Zhou, X.-F.; Kiraly, B.; Wood, J. D.; Alducin, D.; Myers, B. D.; Liu, X.; Fisher, B. L.; Santiago, U.; Guest, J. R.; et al. (December 17, 2015). "Synthesis of borophenes: Anisotropic, two-dimensional boron polymorphs". Science. 350 (6267): 1513–1516. Bibcode:2015Sci...350.1513M. doi:10.1126/science.aad1080. PMC 4922135. PMID 26680195.
- Feng, Baojie; Zhang, Jin; Zhong, Qing; Li, Wenbin; Li, Shuai; Li, Hui; Cheng, Peng; Meng, Sheng; Chen, Lan; Wu, Kehui (March 28, 2016). "Experimental realization of two-dimensional boron sheets". Nature Chemistry. 8 (6): 563–568. arXiv:1512.05029. Bibcode:2016NatCh...8..563F. doi:10.1038/nchem.2491. PMID 27219700.
- Pauling, Linus (1960). The nature of the chemical bond (3rd ed.). Cornell University Press. ISBN 0-8014-0333-2.
- arXiv, Emerging Technology from the. "Sorry, graphene—borophene is the new wonder material that's got everyone excited". MIT Technology Review. Retrieved August 2, 2019.
- Kochaev, A. (October 11, 2017). "Elastic properties of noncarbon nanotubes as compared to carbon nanotubes". Physical Review B. 96 (15): 155428. doi:10.1103/PhysRevB.96.155428.
- Zhang, Z.; Yang, Yang.; Penev, E.S.; Yakobson, B.I. (January 11, 2017). "Elasticity, Flexibility, and Ideal Strength of Borophenes". Advanced Functional Materials. 27 (9): 1605059. arXiv:1609.07533. doi:10.1002/adfm.201605059.
- Boustani, Ihsan (June 15, 1997). "Systematic ab initio investigation of bare boron clusters: Determination of the geometry and electronic structures of Bn (n=2-14)". Physical Review B. 55 (24): 16426–16438. doi:10.1103/PhysRevB.55.16426.
- Boustani, Ihsan (October 1997). "New Convex and Spherical Structures of Bare Boron Clusters". Journal of Solid State Chemistry. 133 (1): 182–189. doi:10.1006/jssc.1997.7424.
- Boustani, I; Quandt, A (September 1, 1997). "Nanotubules of bare boron clusters: Ab initio and density functional study". Europhysics Letters (EPL). 39 (5): 527–532. doi:10.1209/epl/i1997-00388-9.
- Gindulytė, Asta; Lipscomb, William N.; Massa, Lou (December 1998). "Proposed Boron Nanotubes". Inorganic Chemistry. 37 (25): 6544–6545. doi:10.1021/ic980559o. PMID 11670779.
- Quandt, Alexander; Boustani, Ihsan (October 14, 2005). "Boron Nanotubes". ChemPhysChem. 6 (10): 2001–2008. doi:10.1002/cphc.200500205. PMID 16208735.
- Boustani, Ihsan; Quandt, Alexander; Hernández, Eduardo; Rubio, Angel (February 8, 1999). "New boron based nanostructured materials". The Journal of Chemical Physics. 110 (6): 3176–3185. doi:10.1063/1.477976.
- Kunstmann, Jens; Quandt, Alexander (July 12, 2006). "Broad boron sheets and boron nanotubes: An ab initio study of structural, electronic, and mechanical properties". Physical Review B. 74 (3): 035413. arXiv:cond-mat/0509455. doi:10.1103/PhysRevB.74.035413.
- Gonzalez Szwacki, Nevill; Sadrzadeh, Arta; Yakobson, Boris I. (April 20, 2007). "B80 Fullerene: An Ab Initio Prediction of Geometry, Stability, and Electronic Structure". Physical Review Letters. 98 (16): 166804. Bibcode:2007PhRvL..98p6804G. doi:10.1103/PhysRevLett.98.166804. PMID 17501448.
- Tang, Hui & Ismail-Beigi, Sohrab (2007). "Novel Precursors for Boron Nanotubes: The Competition of Two-Center and Three-Center Bonding in Boron Sheets". Physical Review Letters. 99 (11): 115501. arXiv:0710.0593. Bibcode:2007PhRvL..99k5501T. doi:10.1103/PhysRevLett.99.115501. PMID 17930448.
- Özdoğan, C.; Mukhopadhyay, S.; Hayami, W.; Güvenç, Z. B.; Pandey, R.; Boustani, I. (March 18, 2010). "The Unusually Stable B100 Fullerene, Structural Transitions in Boron Nanostructures, and a Comparative Study of α- and γ-Boron and Sheets". The Journal of Physical Chemistry C. 114 (10): 4362–4375. doi:10.1021/jp911641u.
- Zhai, Hua-Jin; Kiran, Boggavarapu; Li, Jun; Wang, Lai-Sheng (November 9, 2003). "Hydrocarbon analogues of boron clusters — planarity, aromaticity and antiaromaticity". Nature Materials. 2 (12): 827–833. doi:10.1038/nmat1012. PMID 14608377.
- Piazza, Z. A.; Hu, H. S.; Li, W. L.; Zhao, Y. F.; Li, J.; Wang, L. S. (2014). "Planar hexagonal B36 as a potential basis for extended single-atom layer boron sheets". Nature Communications. 5: 3113. Bibcode:2014NatCo...5.3113P. doi:10.1038/ncomms4113. PMID 24445427.
- Zhang, L. Z.; Yan, Q. B.; Du, S. X.; Su, G.; Gao, H.-J. (August 15, 2012). "Boron Sheet Adsorbed on Metal Surfaces: Structures and Electronic Properties". The Journal of Physical Chemistry C. 116 (34): 18202–18206. doi:10.1021/jp303616d.
- Liu, Yuanyue; Penev, Evgeni S.; Yakobson, Boris I. (March 11, 2013). "Probing the Synthesis of Two-Dimensional Boron by First-Principles Computations". Angewandte Chemie International Edition. 52 (11): 3156–3159. arXiv:1312.0656. doi:10.1002/anie.201207972. PMID 23355180.
- Liu, Hongsheng; Gao, Junfeng; Zhao, Jijun (November 18, 2013). "From Boron Cluster to Two-Dimensional Boron Sheet on Cu(111) Surface: Growth Mechanism and Hole Formation". Scientific Reports. 3 (1): 3238. doi:10.1038/srep03238. PMC 3831238. PMID 24241341.
- Zhang, Z.; Yang, Y.; Gao, G.; Yakobson, B.I. (September 2, 2015). "Two-Dimensional Boron Monolayers Mediated by Metal Substrates". Angewandte Chemie International Edition. 54 (44): 13022–13026. doi:10.1002/anie.201505425. PMID 26331848.
- Wu, Xiaojun; Dai, Jun; Zhao, Yu; Zhu, Zhiwen; Yang, Jinlong; Zeng, Xiao Cheng (July 20, 2012). "Two-Dimensional Boron Monolayer Sheets". ACS Nano. 6 (8): 7443–7453. doi:10.1021/nn302696v. PMID 22816319.
- Zhang, Z.; Penev, E.S.; Yakobson, B.I. (October 31, 2017). "Two-dimensional boron: structures, properties and applications". Chemical Society Reviews. 46 (22): 6746–6763. doi:10.1039/c7cs00261k. PMID 29085946.
External links
| Look up borophene in Wiktionary, the free dictionary. |
