Blaise ketone synthesis
The Blaise ketone synthesis (named after Edmond Blaise) is the chemical reaction of acid chlorides with organozinc compounds to give ketones.
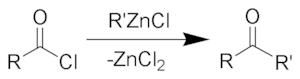
The Blaise ketone synthesis
| Blaise ketone synthesis | |
|---|---|
| Named after | Edmond Blaise |
| Reaction type | Coupling reaction |
The reaction also works with organocuprates.
Variations
Blaise-Maire reaction
The Blaise-Maire reaction is the Blaise ketone synthesis using β-hydroxy acid chlorides to give β-hydroxyketones, which are converted into α,β-unsaturated ketones using sulfuric acid.
gollark: Oh, like our interpreter race.
gollark: Probably just lack of experience and/or poor teaching, but still.
gollark: Some people seem to not really get how stuff, well, generalizes?
gollark: or at least not for a decade or more.
gollark: We're actually up to ES11, and they will **never** drop backward compatibility.
See also
References
- ^ Blaise, E. E.; Koehler, A. (1910). "Synthèse au moyen des dérivés organo-métalliques mixtes du zinc (II)". Bull. Soc. Chim. Fr. 7: 215–227.
- ^ Blaise, E. E. (1911). "Sur les dérivés organo-métalliques mixtes du zinc et leur emploi dans la synthèse organique". Bull. Soc. Chim. Fr. 9: 1.
- ^ Posner, G. H.; Whitten, C. E. (1976). "Secondary and Tertiary Alkyl Ketones from Carboxylic Acid Chlorides and Lithium Phenylthio(Alkyl)Cuprate Reagents: tert-Butyl Phenyl Ketone". Organic Syntheses. 55: 122.; Collective Volume, 6, p. 248
- ^ Fujisawa, T.; Sato, T. (1988). "Ketones from Carboxylic Acids and Grignard Reagents: Methyl 6-Oxodecanoate". Organic Syntheses. 66: 116.; Collective Volume, 8, p. 441
- ^ Cason, J. (1947). "The Use of Organocadmium Reagents for the Preparation of Ketones". Chem. Rev. 40 (1): 17. doi:10.1021/cr60125a002. PMID 20287882.
- ^ Shirley, D. A. (1954). "The Synthesis of Ketones from Acid Halides and Organometallic Compounds of Magnesium, Zinc, and Cadmium". Organic Reactions. 8: 29. doi:10.1002/0471264180.or008.02. ISBN 0471264180.
- ^ Blaise, E. E.; Maire, M. (1907). "Synthèse au moyen des dérivés organo-métalliques mixtes du zinc. Cétones non saturées αβ-acycliques". Compt. Rend. 145: 73-75.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.