Betaine
A betaine (/ˈbiːtə.iːn, bɪˈteɪ-, -ɪn/) in chemistry is any neutral chemical compound with a positively charged cationic functional group such as a quaternary ammonium or phosphonium cation (generally: onium ions) that bears no hydrogen atom and with a negatively charged functional group such as a carboxylate group that may not be adjacent to the cationic site. A betaine is a specific type of zwitterion. Historically, the term was reserved for TMG (trimethylglycine) only. Biologically, TMG is involved in methylation reactions and detoxification of homocysteine.
The pronunciation of the compound reflects its origin and first isolation from sugar beets (Beta vulgaris subsp. vulgaris), and does not derive from the Greek letter beta (β), however, it often is pronounced beta-INE or BEE-tayn.[1]
In biological systems, many naturally occurring betaines serve as organic osmolytes. These are substances synthesized or taken up from the environment by cells for protection against osmotic stress, drought, high salinity, or high temperature. Intracellular accumulation of betaines permits water retention in cells, thus protecting from the effects of dehydration. This accumulation is non-perturbing to enzyme function, protein structure, and membrane integrity. Betaine is also a methyl donor of increasingly recognised significance in biology.
Glycine betaine
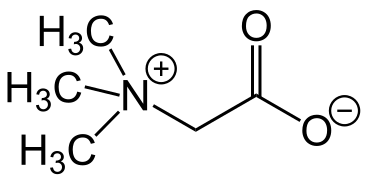
The original betaine, N,N,N-trimethylglycine, was named after its discovery in sugar beet (Beta vulgaris subsp. vulgaris) in the nineteenth century.[2] It is a small N-trimethylated amino acid. It is a zwitterion, which cannot isomerize because there is no labile hydrogen atom attached to the nitrogen atom. This substance may be called glycine betaine to distinguish it from other betaines.
Commercial uses
Phosphonium betaines are intermediates in the Wittig reaction.
The addition of betaine to polymerase chain reactions improves the amplification of DNA by reducing the formation of secondary structure in GC-rich regions. The addition of betaine has been reported to enhance the specificity of the polymerase chain reaction by eliminating the base pair composition dependence of DNA melting.[3][4]
References
- Alex Nickon and Ernest F. Silversmith (1987). Organic Chemistry, the Name Game: Modern Coined Terms and Their Origins. Pergamon. ISBN 978-0080344812.
- DNA Methylation and Complex Human Disease, Michel Neidhart
- Rees, William A.; Yager, Thomas D.; Korte, John; Von Hippel, Peter H. (1993). "Betaine can eliminate the base pair composition dependence of DNA melting". Biochemistry. 32 (1): 137–44. doi:10.1021/bi00052a019. PMID 8418834.
- Henke, W; Herdel, K; Jung, K; Schnorr, D; Loening, SA (1997). "Betaine improves the PCR amplification of GC-rich DNA sequences". Nucleic Acids Research. 25 (19): 3957–8. doi:10.1093/nar/25.19.3957. PMC 146979. PMID 9380524.
Further reading
- IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "betaines". doi:10.1351/goldbook.B00637