Benzylacetone
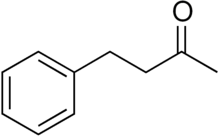
Benzylacetone (IUPAC name: 4-phenylbutan-2-one) is a liquid with a sweet, flowery smell that is considered to be the most abundant attractant compound in flowers (e.g. Coyote Tobacco, Nicotiana attenuata)[1][2] and one of volatile components of cocoa.[3]
 | |
| Names | |
|---|---|
| Preferred IUPAC name
4-Phenylbutan-2-one | |
| Other names
4-Phenyl-2-butanone Methyl 2-phenylethyl ketone | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.018.044 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C10H12O | |
| Molar mass | 148.205 g·mol−1 |
| Density | 0.989 g/mL |
| Melting point | −13 °C (9 °F; 260 K) |
| Boiling point | 235 °C (455 °F; 508 K) |
| Hazards | |
| Flash point | 98 °C (208 °F; 371 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
It can be used as an attractant for melon flies (Bactrocera cucurbitae),[4][5] in perfume,[6] and as an odorant for soap.
It can be prepared by the hydrogenation of benzylideneacetone.
See also
References
- Kessler, D. & Baldwin, I.T. (2007). "Making sense of nectar scents: the effects of nectar secondary metabolites on floral visitors of Nicotiana attenuata". The Plant Journal. 49 (5): 840–854. doi:10.1111/j.1365-313X.2006.02995.x. PMID 17316174. Archived from the original on 2013-01-05.
- Baldwin, I.T.; et al. (1997). "Patterns and Consequences of Benzyl Acetone Floral Emissions from Nicotiana attenuata Plants". J. Chem. Ecol. 23 (100): 2327–2343. doi:10.1023/B:JOEC.0000006677.56380.cd.
- Karl-Georg Fahlbusch, Franz-Josef Hammerschmidt, Johannes Panten, Wilhelm Pickenhagen, Dietmar Schatkowski, Kurt Bauer, Dorothea Garbe & Horst Surburg: Flavors and Fragrances, Ullmann's Encyclopedia of Industrial Chemistry, John Wiley & Sons, New York, 2003. Cited 28.8.2015.
- "University of Florida Featured Creatures". Retrieved 2008-11-18.
- "Answers.com webpage". Retrieved 2008-11-18.
- "The Goods Company webpage". Retrieved 2008-11-18.
External links
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.