Benzenediazonium chloride
Benzenediazonium chloride is an organic compound with the formula [C6H5N2]Cl. It is a salt of a diazonium cation and chloride. It exists as a colourless solid that is soluble in polar solvents including water. It is the parent member of the aryldiazonium compounds,[1] which are widely used in organic chemistry. Because the salt is unstable, it is not commercially available but is prepared upon demand.
 | |
 | |
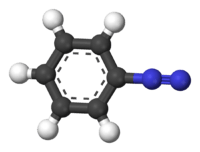 The benzenediazonium ion | |
| Names | |
|---|---|
| IUPAC name
Benzenediazonium chloride | |
| Other names
Phenyldiazonium chloride | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.002.584 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C6H5ClN2 | |
| Molar mass | 140.57 g·mol−1 |
| Appearance | colorless crystals |
| Melting point | decomposes |
| Boiling point | decomposes |
| very good, hydroscopic | |
| Hazards | |
| Main hazards | unstable at temperature exceeding 278K (5C), possibly explosive |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Synthesis
This compound is prepared by diazotization of aniline in the presence of hydrochloric acid:[2] The conversion involves in situ production of nitrous acid (HNO2), which reacts with the aniline:
- C6H5NH2 + HNO2 + HCl → [C6H5N2]Cl + 2 H2O
The reactions are conducted at low temperature to minimize decomposition of the diazonium salt. The diazonium salt is not isolated.
Benzenediazonium tetrafluoroborate
Benzenediazonium tetrafluoroborate can be obtained from crude benzenediazonium chloride by salt metathesis using tetrafluoroboric acid. The tetrafluoroborate is more stable.[2]
Properties
The diazo group (N2) can be replaced by many other groups, usually anions, giving a variety of substituted phenyl derivatives:
- C6H5N2+ + Nu− → C6H5Nu + N2
These transformations are associated with many named reactions including the Schiemann reaction, Sandmeyer reaction, and Gomberg-Bachmann reaction. A wide range of groups that can be used to replace N2 including halide, SH−, CO2H−, OH−. Of considerable practical value in the dye industry are the diazo coupling reactions.
The reaction of phenyldiazonium salts with aniline gives 1,3-diphenyltriazene.[3]
Safety
The compound is explosive.[4]
References
- March, J. (1992). Advanced Organic Chemistry (4th ed.). New York: J. Wiley and Sons. ISBN 0-471-60180-2.
- Flood, D. T. (1933). "Fluorobenzene". Org. Synth. 13: 46. doi:10.15227/orgsyn.013.0046..
- Hartman, W. W.; Dickey, J. B. (1934). "Diazoaminobenzene". Organic Syntheses. 14: 24. doi:10.15227/orgsyn.014.0024.CS1 maint: multiple names: authors list (link)
- Nesmajanow, A. N. (1932). "β-Naphthylmercuric chloride". Organic Syntheses. 12: 54.; Collective Volume, 2, p. 432