BPDA
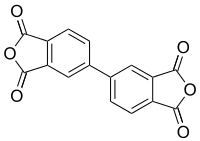
BPDA or biphenyl-tetracarboxylic acid dianhydride is a monomer used in the production of some polyimides.
 | |
| Names | |
|---|---|
| IUPAC name
5,5’-Bi-2-benzofuran-1,1’,3,3’-tetrone | |
| Other names
3,3',4,4'-Biphenyltetracarboxylic dianhydride; 4,4'-Biphthalic dianhydride | |
| Identifiers | |
3D model (JSmol) |
|
| Abbreviations | BPDA |
| ChemSpider | |
| ECHA InfoCard | 100.017.585 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C16H6O6 | |
| Molar mass | 294.218 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Applications
- Tape automated bonding (TAB), chip on film (COF), lead lock tape, high density flexible printed circuit (FPC), stiffener for FPC, office automation equipment, flexible solar cells, speaker diaphragms (for mobile phones, plasma televisions and car audio, etc.), heavy electric machinery, office automation equipment, thermal control film for satellites, printed circuit boards, metallic substrates, sheet heating elements, heat resistance wires.
Characteristics
- Physical, mechanical, electrical, and chemical properties under high-temperature conditions.
- High tensile strength and modulus, and also features outstanding long-term heat resistance.
Analytics
The chemical shifts in 1H and 13C NMR spectroscopy are given in the literature.[1] The melting point is 299 - 301 °C.[1]
gollark: You can fit Shrek in *very* low quality in 8MiB!
gollark: The amazing power of MODERN CODECS™.
gollark: Well, they seem to have tried to do that initially for a bit, then gave up (at least for my year), leaving us doing something like two lessons a week but being set a bunch of random papers to do (which I mostly ignored because they are extremely boring).
gollark: I'm glad school is over for me now and I can avoid worrying about them doing stupid things.
gollark: https://archlinuxarm.org/platforms/armv8/broadcom/raspberry-pi-3
References
- "An Efficient Synthetic Method for 3,3',4,4'-Biphenyltetracarboxylic Anhydride". Bulletin of the Korean Chemical Society. 30 (9): 2161–2164. doi:10.5012/bkcs.2009.30.9.2161.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.