Chloroalkyl ether
Chloroalkyl ethers are a class of organic compounds with the general structure R-O-(CH2)n-Cl, characterized as an ether connected to a chloromethyl group via an alkane chain.
methylether.svg.png)
Chloromethyl methyl ether (CMME) is an ether with the formula CH3OCH2Cl. It is used as an alkylating agent and industrial solvent to manufacture dodecylbenzyl chloride, water repellents, ion-exchange resins, polymers, and as a chloromethylation reagent. It is a known human carcinogen.[1] In organic synthesis the compound is used for the introduction of the methoxymethyl (MOM) protecting group.
Closely related compounds of industrial importance are bis(chloromethyl) ether (BCME) (closely related to chemical weapon sulfur mustard)[2] and benzyl chloromethyl ether (BOMCl).
| Chloromethyl ether | R | Molar mass | CAS number | Boiling point °C | |
| Benzyl chloromethyl ether | Benzyl | 156.61 | 3587-60-8 | 102 °C @ 14 mmHg (1.9 kPa) | |
| Chloromethyl methyl ether | Methyl | 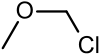 | 80.51 | 107-30-2 | 55-57 |
| Bis(chloromethyl) ether | 114.96 | 542-88-1 | 106 | ||
| tert-Butyl chloromethyl ether | Butyl | 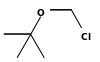 | 124.5 | ||
| Methoxyethyl chloromethyl ether | 124.57 | 3970-21-6 | 50-52 °C @ 13 mmHg (1.7 kPa) | ||
| Dichloromethyl methyl ether | 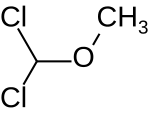 | 114.96 | 4885-02-3 | 82 - 85.5 °C | |
| Representative chloroalkyl ethers[3] | |||||
Alcohol protection
2-Methoxyethoxymethyl (MEM) group is commonly used in organic synthesis as a protecting group for alcohols.

Most common protection methods
- Treatment of alcohol with bases such as sodium hydride or potassium hydride and 2-methoxyethoxymethyl chloride in tetrahydrofuran (THF) at 0 °C[4]
- MEM group can also be installed at ambient temperature with 2-methoxyethoxymethyl chloride and a mild base such as N,N-diisopropylethylamine (DIPEA) in dichloromethane[5]
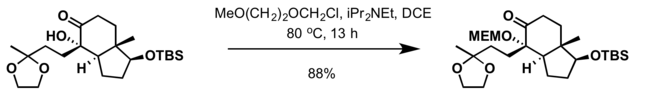
Most common deprotection methods
The 2-methoxyethoxymethyl protecting group can be cleaved with a range of Lewis acids, including but not limited to:
- TiCl4 or ZnBr2 in dichloromethane at 0 °C to ambient temperature
- If the solvent of choice is a protic solvent such as methanol, formic acid can be used to cleave MEM group at elevated temperatures
_____________________________
Methoxymethyl (MOM) is used as a protecting group for alcohols in organic synthesis.
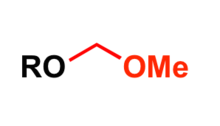
Most common protection methods
- Treatment of alcohol with N,N-diisopropylethylamine (DIPEA) and methoxymethyl chloride (MOM chloride) in dichloromethane at 0 °C[6]

- For reactions carried out in more polar solvents such as tetrahydrofuran (THF) or N,N-dimethylformamide (DMF), protection of alcohol can be carried out using sodium hydride at 0 °C to ambient temperatures
Most common deprotection methods
MOM group can be cleaved with acid, commonly used conditions for deprotection of MOM alcohols include:[6]
- Concentrated hydrochloric acid in methanol or water
- Trifluoroacetic acid (TFA) in dichloromethane
- Acetyl chloride in methanol at 0 °C
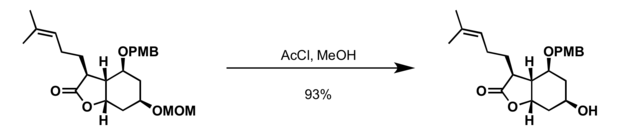
References
- Bis(chloromethyl) Ether and Technical-Grade Chloromethyl Methyl Ether CAS Nos. 542-88-1 and 107-30-2, Report on carcinogens, Eleventh edition
- Bis(Chloromethyl) ether Safety Data Sheet, Division of Occupational Health and Safety, US National Institutes of Health
- "Sigma-Aldrich: Analytical, Biology, Chemistry & Materials Science products and services".
- Corey, E. J.; Gras, Jean-Louis; Ulrich, Peter (1976-03-01). "A new general method for protection of the hydroxyl function". Tetrahedron Letters. 17 (11): 809–812. doi:10.1016/S0040-4039(00)92890-9.
- Lee, Hong Myung; Nieto-Oberhuber, Cristina; Shair, Matthew D. (2008-12-17). "Enantioselective Synthesis of (+)-Cortistatin A, a Potent and Selective Inhibitor of Endothelial Cell Proliferation". Journal of the American Chemical Society. 130 (50): 16864–16866. doi:10.1021/ja8071918. ISSN 0002-7863. PMID 19053422.
- Wuts, Peter G. M.; Greene, Theodora W. (2006). Greene's Protective Groups in Organic Synthesis, Fourth Edition - Wuts - Wiley Online Library. doi:10.1002/0470053488. ISBN 9780470053485.
- Enders, Dieter; Geibel, Gunter; Osborne, Simon (2000-04-17). "Diastereo- and Enantioselective Total Synthesis of Stigmatellin A". Chemistry – A European Journal. 6 (8): 1302–1309. doi:10.1002/(SICI)1521-3765(20000417)6:8<1302::AID-CHEM1302>3.0.CO;2-J.
- Amano, Seiji; Takemura, Noriaki; Ohtsuka, Masami; Ogawa, Seiichiro; Chida, Noritaka (1999-03-26). "Total synthesis of paniculide A from d-glucose". Tetrahedron. 55 (13): 3855–3870. doi:10.1016/S0040-4020(99)00096-4.