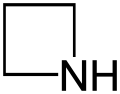
Azetidine
Azetidine is a saturated heterocyclic organic compound containing three carbon atoms and one nitrogen atom. It is a liquid at room temperature with a strong odor of ammonia and is strongly basic compared to most secondary amines.
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Azetidine[1] | |||
| Systematic IUPAC name
Azacyclobutane | |||
| Other names
Azetane Trimethylene imine 1,3-Propylenimine | |||
| Identifiers | |||
3D model (JSmol) |
|||
| 102384 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.007.240 | ||
| EC Number |
| ||
| 986 | |||
PubChem CID |
|||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C3H7N | |||
| Molar mass | 57.09 g/mol | ||
| Appearance | colorless liquid | ||
| Density | 0.847 g/cm3 at 25 °C | ||
| Boiling point | 61 to 62 °C (142 to 144 °F; 334 to 335 K) | ||
| miscible | |||
| Acidity (pKa) | 11.29 (conjugate acid; H2O)[2] | ||
| Hazards | |||
| Main hazards | Somewhat strong base, combustible | ||
| GHS pictograms |   | ||
| GHS Signal word | Danger | ||
GHS hazard statements |
H225, H314 | ||
| P210, P233, P240, P241, P242, P243, P260, P264, P280, P301+330+331, P303+361+353, P304+340, P305+351+338, P310, P321, P363, P370+378, P403+235, P405, P501 | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Synthesis and occurrence
Azetidines can be prepared by reduction of azetidinones (β-lactams) with lithium aluminium hydride. Even more effective is a mixture of lithium aluminium hydride and aluminium trichloride, a source of "AlClH2" and "AlCl2H".[3] Azetidine can also be produced by a multistep route from 3-amino-1-propanol.[4]
Regio- and diastereoselective synthesis of 2-arylazetidines could be performed from appropriately substituted oxyranes via ring transformation. It is controlled by Baldwin’s Rules with remarkable functional group tolerance.
Azetidine and its derivatives are relatively rare structural motifs in natural products. They are a component of mugineic acids and penaresidins. Perhaps the most abundant azetidine containing natural product is azetidine-2-carboxylic acid, a toxic homolog of proline.[5]
See also
- Azete, the unsaturated analog
References
- "Front Matter". Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 147. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- Haynes, William M., ed. (2016). CRC Handbook of Chemistry and Physics (97th ed.). CRC Press. p. 5–89. ISBN 978-1498754286.
- Alcaide, Benito; Almendros, Pedro; Aragoncillo, Cristina (2007). "Β-Lactams: Versatile Building Blocks for the Stereoselective Synthesis of Non-β-Lactam Products". Chemical Reviews. 107 (11): 4437–4492. doi:10.1021/cr0307300. PMID 17649981.
- Donald H. Wadsworth (1973). "Azetidine". Organic Syntheses. 53: 13. doi:10.15227/orgsyn.053.0013.
- Kovács, Ervin; Ferenc, Faigl; Zoltan, Mucsi (Aug 10, 2020). "Regio- and Diastereoselective Synthesis of 2-Arylazetidines. Quantum Chemical Explanation of Baldwin's Rules for the Ring-formation Reactions of Oxiranes". Journal of Organic Chemistry. doi:10.1021/acs.joc.0c01310. Retrieved Aug 10, 2020.