Auwers synthesis
The Auwers synthesis is a series of organic reactions forming a flavonol from a coumarone. This reaction was first reported by Karl von Auwers in 1908.[1][2][3][4][5]
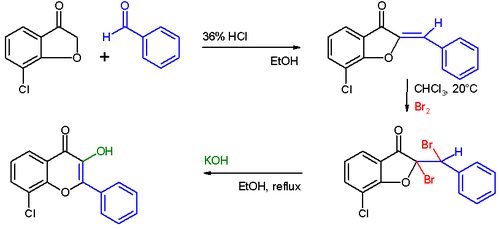
The Auwers synthesis
| Auwers synthesis | |
|---|---|
| Named after | Karl von Auwers |
| Reaction type | Coupling reaction |
| Identifiers | |
| RSC ontology ID | RXNO:0000474 |
The first step in this procedure is an acid catalyzed aldol condensation between benzaldehyde and a 3-cyclooxapentanone to an o-hydroxychalcone. Bromination of the alkene group gives a dibromo-adduct which rearranges to the flavonol by reaction with potassium hydroxide.
Mechanism
A possible mechanism for the rearrangement step is shown below:
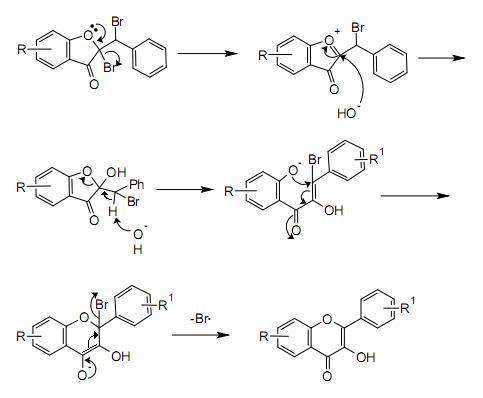
Mechanism
gollark: Most of it is just stuff like```Uf=g9```
gollark: Actually, no.
gollark: It halts periodically because printing is occuring so fast that the tray fills up in the two seconds between clearing cycles.
gollark: I filter out code beginning with `#`, so try again.
gollark: Technically, most of what gets printed is *code*, and most of it in fact runs fine with no runtime errors.
References
- K. Auwers, K. Müller, "Umwandlung von Benzal-cumaranonen in Flavonole", Ber. Dtsch. Chem. Ges., 41, 4233–4241 (1908) (doi:10.1002/cber.190804103137).
- K. v. Auwers, P. Pohl, "Über die Umwandlung von Benzalcumaranonen in Flavonole", Liebigs Ann. Chem., 405, 243–294 (1914) (doi:10.1002/jlac.19144050302).
- K. v. Auwers, P. Pohl, "Eine Synthese des Fisetins", Ber. Dtsch. Chem. Ges., 48, 85–90 (1915) (doi:10.1002/cber.19150480114).
- K. v. Auwers, "Zur Bildung von Flavonolen aus Benzal-cumaranonen", Ber. Dtsch. Chem. Ges., 49, 809–819 (1916) (doi:10.1002/cber.19160490188).
- K. v. Auwers, E. Auffenberg, "Über Cumaranone und Hydrindone", Ber. Dtsch. Chem. Ges., 52, 92-113 (1919) (doi:10.1002/cber.19190520114).
See also
- Algar-Flynn-Oyamada reaction
- Allan-Robinson reaction
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.