Autofluorescence
Autofluorescence is the natural emission of light by biological structures such as mitochondria and lysosomes when they have absorbed light, and is used to distinguish the light originating from artificially added fluorescent markers (fluorophores).[1]

The most commonly observed autofluorescencing molecules are NADPH and flavins; the extracellular matrix can also contribute to autofluorescence because of the intrinsic properties of collagen and elastin.[1]
Generally, proteins containing an increased amount of the amino acids tryptophan, tyrosine and phenylalanine show some degree of autofluorescence.[2]
Autofluorescence also occurs in non-biological materials found in many papers and textiles. Autofluorescence from U.S. paper money has been demonstrated as a means for discerning counterfeit currency from authentic currency.[3]
Microscopy
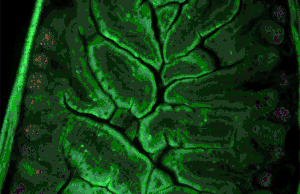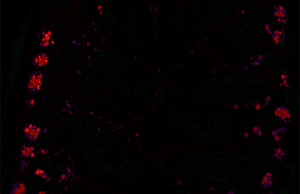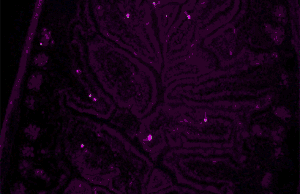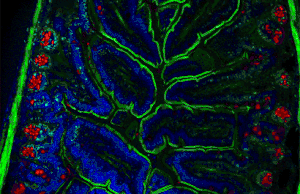
Autofluorescence can be problematic in fluorescence microscopy. Light-emitting stains (such as fluorescently labelled antibodies) are applied to samples to enable vizualisation of specific structures.
Autofluorescence interferes with detection of specific fluorescent signals, especially when the signals of interest are very dim — it causes structures other than those of interest to become visible.
In some microscopes (mainly confocal microscopes), it is possible to make use of different lifetime of the excited states of the added fluorescent markers and the endogenous molecules to exclude most of the autofluorescence.
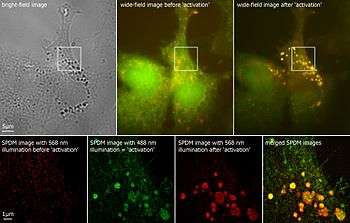
In a few cases, autofluorescence may actually illuminate the structures of interest, or serve as a useful diagnostic indicator.[1]
For example, cellular autofluorescence can be used as an indicator of cytotoxicity without the need to add fluorescent markers.[4]
The autofluorescence of human skin can be used to measure the level of advanced glycation end-products (AGEs), which are present in higher quantities during several human diseases.[5]
Optical imaging systems that utilize multispectral imaging can reduce signal degradation caused by autofluorescence while adding enhanced multiplexing capabilities.[6]
The super resolution microscopy SPDM revealed autofluorescent cellular objects which are not detectable under conventional fluorescence imaging conditions.[7]
Autofluorescent molecules

| Molecule |
Excitation (nm) |
Fluorescence (nm) Peak |
Organisms |
Reference |
| NAD(P)H | 340 | 450 | All | [8] |
| Chlorophyll | 465 / 665 | 673 / 726 | Plants | |
| Collagen | 270-370 | 305-450 | Animals | [8] |
| Retinol | 500 | Animals & bacteria | [9] | |
| Riboflavin | 550 | All | [9] | |
| Cholecalciferol | 380-460 | Animals | [9] | |
| Folic acid | 450 | All | [9] | |
| Pyridoxine | 400 | All | [9] | |
| Tyrosine | 270 | 305 | All | [2] |
| Dityrosine | 325 | 400 | Animals | [2] |
| Excimer-like aggregate | 270 | 360 | Animals | collagen[2] |
| Glycation adduct | 370 | 450 | Animals | [2] |
| Indolamine | Animals | |||
| Lipofuscin | 410-470 | 500-695 | Eukaryotes | [10] |
| Lignin, a polyphenol | 335 / 488 | 455 / 535 | Plants | [11] |
| Tryptophan | 280 | 300-350 | All | |
| Flavin | 380-490 | 520-560 | All | |
| Melanin | 340–400 | 360–560 | Animals | [12] |
See also
References
- Monici M. (2005). Cell and tissue autofluorescence research and diagnostic applications. Biotechnol Annu. Rev. Biotechnology Annual Review. 11. pp. 227–56. doi:10.1016/S1387-2656(05)11007-2. ISBN 9780444519528. PMID 16216779.
- Julian M. Menter (2006). "Temperature dependence of collagen fluorescence". Photochem. Photobiol. Sci. 5 (4): 403–410. doi:10.1039/b516429j. PMID 16583021.
- Chia, Thomas; Michael Levene (17 November 2009). "Detection of counterfeit U.S. paper money using intrinsic fluorescence lifetime". Optics Express. 17 (24): 22054–22061. doi:10.1364/OE.17.022054. PMID 19997451.
- Fritzsche M, Mandenius CF (September 2010). "Fluorescent cell-based sensing approaches for toxicity testing". Anal Bioanal Chem. 398 (1): 181–91. doi:10.1007/s00216-010-3651-6. PMID 20354845. S2CID 22712460.
- Gerrits EG, Smit AJ, Bilo HJ (March 2009). "AGEs, autofluorescence and renal function". Nephrol. Dial. Transplant. 24 (3): 710–3. doi:10.1093/ndt/gfn634. PMID 19033250. Retrieved 2011-04-23.
- James R. Mansfield, Kirk W. Gossage, Clifford C. Hoyt, and Richard M. Levenson "Autofluorescence removal, multiplexing, and automated analysis methods for in-vivo fluorescence imaging" J. Biomed. Opt., Vol. 10, 041207 (2005)
- Kaufmann R, Müller P, Hausmann M, Cremer C (2010). "Imaging label-free intracellular structures by localisation microscopy". Micron. 42 (4): 348–352. doi:10.1016/j.micron.2010.03.006. PMID 20538472.
- Georgakoudi I, Jacobson BC, Müller MG, Sheets EE, Badizadegan K, Carr-Locke DL, Crum CP, Boone CW, Dasari RR, Van Dam J, Feld MS (2002-02-01). "NAD(P)H and collagen as in vivo quantitative fluorescent biomarkers of epithelial precancerous changes". Cancer Res. 62 (3): 682–687. PMID 11830520.
- Zipfel WR, Williams RM, Christie R, Nikitin AY, Hyman BT, Webb WW (2003-06-10). "Live tissue intrinsic emission microscopy using multiphoton-excited native fluorescence and second harmonic generation". Proceedings of the National Academy of Sciences of the United States of America. 100 (12): 7075–7080. doi:10.1073/pnas.0832308100. PMC 165832. PMID 12756303.
- Schönenbrücher, Holger; et al. (2008). "Fluorescence-Based Method, Exploiting Lipofuscin, for Real-Time Detection of Central Nervous System Tissues on Bovine Carcasses". Journal of Agricultural and Food Chemistry. 56 (15): 6220–6226. doi:10.1021/jf0734368. PMID 18620407.
- Llyod Donaldson; Nari Williams (February 2018). "Imaging and Spectroscopy of Natural Fluorophores in Pine Needles". Plants. 7 (1): 10. doi:10.3390/plants7010010. PMC 5874599. PMID 29393922.
- James M. Gallas & Melvin Eisner (May 1987). "Fluorescence of Melanin-Dependence upon Excitation Wavelength and Concentration". Photochem. Photobiol. 45 (5): 595–600. doi:10.1111/j.1751-1097.1987.tb07385.x.