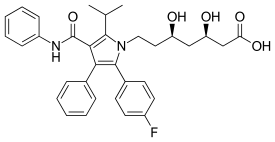
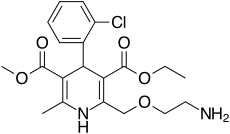
Atorvastatin/amlodipine
The drug combination atorvastatin/amlodipine (brand names Caduet in the United States, Australia, and Russia; and Envacar elsewhere) is a medication approved by the Food and Drug Administration (FDA) for the treatment of high cholesterol and high blood pressure. It is a fixed-dose combination drug containing a HMG-CoA reductase inhibitor and a calcium channel blocker being marketed by the pharmaceutical company Pfizer.[1][2]
 | |
 | |
| Combination of | |
|---|---|
| Amlodipine | Calcium channel blocker |
| Atorvastatin | Statin |
| Clinical data | |
| Trade names | Caduet, Envacar |
| License data | |
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| PubChem CID | |
| ChemSpider | |
| (verify) | |
References
- "CADUET (amlodipine besylate/atorvastatin calcium) Tablets" (PDF). NDA 21-540/S-009. United States Food and Drug Administration. February 2010. Retrieved 2010-09-25.
- Devabhaktuni M, Bangalore S (2009). "Fixed combination of amlodipine and atorvastatin in cardiovascular risk management: patient perspectives". Vascular Health and Risk Management. 5 (1): 377–87. doi:10.2147/vhrm.s3339. PMC 2686256. PMID 19475775.
External links
- "Amlodipine Besylate mixture with Atorvastatin Calcium". Drug Information Portal. U.S. National Library of Medicine.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.