Ascocoryne sarcoides
Ascocoryne sarcoides is a species of fungus in the family Helotiaceae. The species name is derived from the Greek sarkodes (fleshy). Formerly known as Coryne sarcoides, its taxonomical history has been complicated by the fact that it may adopt both sexual and asexual forms. Colloquially known as jelly drops[1] or the purple jellydisc,[2] this common fungus appears as a gelatinous mass of pinkish or purple-colored discs. Distributed widely in North America, Europe and Asia, A. sarcoides is a saprobic fungus and grows in clusters on the trunks and branches of a variety of dead woods. Field studies suggest that colonization by A. sarcoides of the heartwood of black spruce confers some resistance to further infection by rot-causing fungi. A. sarcoides contains the antibiotic compound ascocorynin, shown in the laboratory to inhibit the growth of several Gram-positive bacteria.
| Ascocoryne sarcoides | |
|---|---|
 | |
| Scientific classification | |
| Kingdom: | |
| Division: | |
| Class: | |
| Order: | |
| Family: | |
| Genus: | |
| Species: | A. sarcoides |
| Binomial name | |
| Ascocoryne sarcoides | |
| Synonyms | |
Taxonomy

The taxonomical history of this fungus has been complicated by the fact that its life cycle allows for both an imperfect (making asexual spores, or conidia) or perfect (making sexual spores) form; at various times authors have assigned names to one or the other form, but these names have often been at odds with the accepted rules of fungal nomenclature. It was originally described in 1781 by the Dutch scientist Nikolaus Joseph von Jacquin as Lichen sarcoides.[3] Christian Hendrik Persoon called it Peziza sarcoides in 1801. Elias Magnus Fries, in his 1822 publication Systema Mycologicum,[4] described the imperfect state of the fungus under the name Tremella sarcoides. The genus name Coryne was first used in 1851 by Bonorden, who proposed Coryne sarcoides for the imperfect state; in 1865 the Tulasne brothers (Charles and Louis René) used Coryne to refer to both the perfect and imperfect forms. It was designated the type species for the genus in a 1931 publication by Clements and Shear.
Several decades later it became apparent that the name Coryne sarcoides violated the naming conventions imposed by fungal taxonomists—specifically, the species was named after the imperfect state, so in 1967, Groves and Wilson proposed the new genus name Ascocoryne to accommodate the perfect state.[5] The conidial state of this fungus is Coryne dubia Persoon ex S.F. Gray (synonymous with Pirobasidium sarcoides von Hoehnel).[6] The specific epithet is derived from Greek and means "fleshy, flesh-like", from σάρξ (sarx, sarc- in compounds), "flesh", and the common adjectival ending -οειδής (-oeides), "similar, -like".[7]
Description

This fungus is characterized by a fruiting body (technically an apothecia) with a pinkish-purple color and more or less gelatinous consistency. The apothecia, typically 0.5 to 1.5 centimetres (0.2 to 0.6 in) in diameter, start with a roughly spherical shape, then eventually flatten out to become shallowly cup-shaped with a wavy edge and smooth upper surface. The lower surface may be covered with small particles (granular), and the apothecia are either attached directly to the growing surface (sessile), or have a rudimentary stem.[8] The apothecia are accompanied by a conidial form, where non-sexual spores are generated. The conidial form consists of sporodochia, a cushion-like asexual fruiting body mass consisting of short conidiophores (specialized stalks that bear conidia). The sporodochia are similar in color and consistency to the apothecia but very variable in shape, typically club-, spoon-, or tongue-shaped, and bearing minute, cylindrical, straight or curved conidia.[5] As the fungus matures and the apothecia enlarge and press against each other, the apothecia coalesce to form a gelatinous, irregular mass.[9] The flesh, similar to the appearance of the fungus, is pinkish-purple and gelatinous. The odor and taste of A. sarcoides are not distinctive.[8] Ascocoryne sarcoides is not considered edible.[8]
Microscopic features
The spores are translucent (hyaline), smooth, have an ellipsoid shape, with dimensions of 12–16 by 3–5 µm. Spores contain one or two oil droplets. The imperfect (conidial) form of the fungus produces smooth, hyaline spores that are 3–3.5 by 1–2 µm.[10] The asci – sexual spore-bearing cells – have a cylindrical shape, with dimensions of 115–125 by 8–10 µm. The paraphyses (sterile filamentous cells interspersed among the asci) are cylindrical with slightly swollen tips, and few branches.[8]
Similar species
Ascocoryne cylichnium, another small and gelatinous violet-colored species, has apothecia that are more often cup-shaped, and has larger spores—20–24 by 5.5–6 µm.[8] Because of its resemblance to the jelly fungi, A. sarcoides has been mistaken for the basidiomycete species Auricularia auricula and Tremella foliacea. T. foliacea is larger, brown, and leafy in appearance. Auricularia auricula is also larger, typically brown, is disc- or ear-shaped, with a ribbed undersurface. Microscopically, Tremella foliacea and Auricularia auricula are easily distinguished from A. sarcoides by the presence of basidia (rather than asci).[10]
Habitat and distribution
This species has a broad distribution in forested areas of North America and Europe. A saprobic fungus, it derives nutrients from decaying organic matter, and as such is usually found growing on the stumps and logs of fallen deciduous trees. However, it is also found on a variety of living trees as well. For example, in Europe it has been found on the stems of living spruce (Picea abies) in Finland,[11] France,[12] Great Britain,[13] Norway,[14] and Germany.[15]
Other collections sites include Australia,[16] Chile,[17] China,[18] Cuba,[19] Iceland,[20] Korea,[21] and Taiwan.[22] In Hawaii, it grows on trunks of fallen Cibotium[23] and Aleurites trees.[24] A. sarcoides occurs most frequently in late summer and autumn.[8]
Role in tree decay
A number of field studies conducted in the boreal forest region of Northern Ontario (Canada) showed that A. sarcoides was found to be frequently associated with various deciduous and coniferous tree hosts that had been affected by the fungal disease known as heart rot; this discovery was noted as unusual, as most fungal tree infections are known to be caused by Basidiomycetes, not Ascomycetes.[25][26][27][28] In the case of the commercially valuable tree species black spruce (Picea mariana), it was determined that prior colonization by A. sarcoides reduces the incidence of subsequent infection by common fungal pathogens, such as Fomes pini and Scytinostroma galactina; furthermore, A. sarcoides can exist in the wood with no noticeable harmful effects on the host.[25] A similar relationship was shown later to exist with jack pine trees (species Pinus banksiana), whereby A. sarcoides inhibited Peniophora pseudopini, but had little effect on the subsequent growth of Fomes pini.[29] The study also showed that A. sarcoides is isolated more frequently from defective wood as the age of the tree increases (trees examined in the study were over 80 years old), and that it can infect both uninfected heartwood as well as previously decayed wood; in the latter case it usually coexists with the causal fungi.
Bioactive compounds
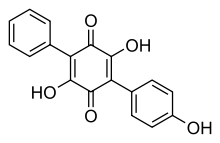
Terphenylquinones are chemical compounds that are widely distributed among the fungi. Ascocoryne sarcoides has been shown to contain a terphenylquinone named ascocorynin—a chemical derivative of the compound benzoquinone. This pigment, when in alkaline solution, turns a dark violet, similar in color to the fruit bodies of the fungus. Ascocorynin has moderate antibiotic activity, and was shown in laboratory tests to inhibit the growth of several Gram-positive bacteria, including the widely distributed food spoilage organism Bacillus stearothermophilus; however, it has no effect on the growth on Gram-negative bacteria, nor does it have any anti-fungal activity.[6]
Volatile organic compounds
In 2008, an isolate of A. sarcoides was observed to produce a series of volatiles including 6 to 9 carbon alcohols, ketones and alkanes.[30] This mixture was called "Mycodiesel" because of its similarity to some existing fuel mixtures. The researchers have suggested that this, combined with its ability to digest cellulose, make it a potential source of biofuel.[31] The isolate was originally identified at Gliocladium roseum but its taxonomy was later revised to Ascococoryne sarcoides.[32] Its genome was sequenced in 2012 in an effort to determine the genetic basis for the production of these volatiles.[33]
References
- Hall JH, Cassidy FG (1985). Dictionary of American regional English. Cambridge: Belknap Press of Harvard University Press. p. 114. ISBN 978-0-674-20519-2.
- "Purple Jellydisc - Ascocoryne sarcoides". Wild About Britain. Archived from the original on 2008-12-03. Retrieved 2009-06-18.
- Jacquin NJ. (1781). Miscellanea austriaca ad botanicum, chemiam et historiam naturalem spectantia (in Latin). 2. p. 20.
- "nzfungi.landcareresearch.co.nz". NZFUNGI - New Zealand Fungi (and Bacteria). New Zealand Landcare Research. Retrieved 2010-01-15.
- Groves JW, Wilson DE (1967). "The nomenclatural status of Coryne". Taxon. 16 (1): 35–41. doi:10.2307/1217104. JSTOR 1217104.
- Quack W, Scholl H, Budzikiewicz H (1982). "Ascorynin, a terphenylquinone from Ascocoryne sarcoides". Phytochemistry. 21 (12): 2921–23. doi:10.1016/0031-9422(80)85069-2.
- Stearn WT. (1973). Botanical Latin (2nd annot. and rev. ed.). Newton Abbot: David & Charles. pp. 265–266, 278.
- Jordan M. (2004). The Encyclopedia of Fungi of Britain and Europe. London: Frances Lincoln. p. 64. ISBN 978-0-7112-2378-3.
- Emberger G. "Ascocoryne sarcoides". Fungi on Wood. Messiah College. Retrieved 2009-06-18.
- Wood M, Stevens F. "Ascocoryne sarcoides". California Fungi. MycoWeb. Archived from the original on 28 June 2009. Retrieved 2009-06-18.
- Kallio T, Tamminen P (1974). "Decay of spruce (Picea abies [L.] Karst.) in the Åland Islands". Acta Forestalia Fennica. 138: 1–42.
- Delatour C.; Sylvestre, Gilberte (1976). "Microflore interne de tissus ligneux de l'epicéa commun sur pied". Annales des Sciences Forestières (in French). 33 (4): 199–219. doi:10.1051/forest/19760402.
- Pawsey RG. (1971). "Some recent observations on decay of conifers associated with extraction damage, and on butt rot caused by Polyporus sehweinitzii and Sparassis crispa". Quarterly Journal of Forestry. 65: 193–208.
- Roll-Hansen F, Roll-Hansen H (1976). "Microflora of sound-looking wood in Picea abies stems". European Journal of Forest Pathology. 9 (5): 308–16. doi:10.1111/j.1439-0329.1979.tb00693.x.
- Zycha H, Dimitri L (1968). "Ausmaß und Ursache der Kernfäule in einer Fichtenprobefläche in Reinhausen (Niedersachsen)". Forstwiss. CBL. (in German). 87 (1): 331–41. doi:10.1007/BF02735875.
- Fuhrer BA. (1985). A Field Companion to Australian Fungi. Fitzroy, Vic., Australia: Five Mile Press. pp. 144–45. ISBN 978-0-86788-063-2.
- Stinson, M., Ezra, D., Hess, W.M., Sears, J., and Strobel, G. (2003). An endophytic Gliocladium sp. of Eucryphia cordifolia producing selective volatile antimicrobial compounds. Plant Science 165, 913–922.
- Zhuang W-Y. (1999). "Discomycetes of tropical China. VI. Additional species from Guangxi" (PDF). Fungal Diversity. 3: 187–96.
- Dennis RWG. (1954). "Some inoperculate Discomycetes of Tropical America". Kew Bulletin. 9 (2): 289–348. doi:10.2307/4114399. JSTOR 4114399.
- Hallgrimsson H, Gotzsche HF (1990). "Notes on Ascomycetes. II. Discomycetes". Acta Botanica Islandica. 10: 31–36.
- Lee JB, Kim SC, Oh DC (2000). "Higher fungal flora of Jeju-do (3): Unrecorded fungi (Ascomycota)". Mycobiology (Meeting abstract). 28 (4): 232–32.
- Liou SC, Chen ZC (1977). "Notes on Taiwan discomycetes. Part I: Pezizales and Helotiales". Tawainia. 22: 29–43.
- Gilbertson RL, Hemmes DE (1997). "Notes on fungi on Hawaiian tree ferns". Mycotaxon. 62: 465–87.
- Cash EK. (1938). "New records of Hawaiian discomycetes". Mycologia. 38 (1): 97–107. doi:10.2307/3754484. JSTOR 3754484.
- Basham JT. (1973). "Heart rot of black spruce in Ontario. I. Stem rot, hidden rot, and management considerations". Canadian Journal of Forest Research. 3 (1): 95–104. doi:10.1139/x73-014.
- Etheridge DE. (1970). "Ascocoryne sarcoides (Jacq. ex Gray) Groves and Wilson and its association with decay of conifers". Fonds Rech. For. Univ. Laval. 13: 19–26.
- Basham JT. (1973). "Heart rot of black spruce in Ontario. 2. Mycoflora in defective and normal wood of living trees". Canadian Journal of Botany. 51 (7): 1379–92. doi:10.1139/b73-173.
- Etheridge DE, Morin LA (1967). "The microbiological condition of wood of living balsam fir and black spruce in Quebec" (PDF). Canadian Journal of Botany. 45 (7): 1003–10. doi:10.1139/b67-104. Retrieved 2010-01-15.
- Basham JT. (1975). "Heart rot of Jack Pine in Ontario. IV. Heartwood-inhabiting fungi, their entry and interactions with living tree" (PDF). Canadian Journal of Forest Research. 5 (4): 706–21. doi:10.1139/x75-097. Retrieved 2010-01-15.
- Strobel, G.A.; Knighton, B.; Kluck, K.; Ren, Y.; Livinghouse, T.; Griffin, M.; Spakowicz, D.; Sears, J. (2008). "The production of myco-diesel hydrocarbons and their derivatives by the endophytic fungus Gliocladium roseum (NRRL 50072)". Microbiology. 154 (11): 3319–28. doi:10.1099/mic.0.2008/022186-0. PMID 18957585.
- "Fungus 'manufactures diesel'". Press Association. 2008-11-04. Retrieved 2008-11-04.
- Griffin, MA; Spakowicz, DJ; Gianoulis, TA; Strobel, SA (Dec 2010). "Volatile organic compound production by organisms in the genus Ascocoryne and a re-evaluation of myco-diesel production by NRRL 50072". Microbiology. 156 (12): 3814–29. doi:10.1099/mic.0.041327-0. PMID 20705658.
- Gianoulis, T.A.; Griffin, M.A.; Spakowicz, D.J.; Dunican, B.F.; Alpha, C.J.; Sboner, A.; Sismour, A.M.; Kodira, C.; Egholm, M.; Church, G.M.; et al. (2012). "Genomic Analysis of the Hydrocarbon-Producing, Cellulolytic, Endophytic Fungus Ascocoryne sarcoides". PLoS Genet. 8 (3): e1002558. doi:10.1371/journal.pgen.1002558. PMC 3291568. PMID 22396667.
External links
| Wikimedia Commons has media related to Ascocoryne sarcoides. |
