Aromadendrin
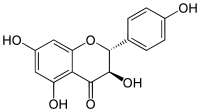
Aromadendrin (aromodedrin or dihydrokaempferol) is a flavanonol, a type of flavonoid. It can be found in the wood of Pinus sibirica.[1]
 | |
| Names | |
|---|---|
| IUPAC name
(2R,3R)-3,5,7-trihydroxy-2-(4-hydroxyphenyl)-2,3-dihydrochromen-4-one | |
| Other names
Aromadedrin Dihydrokaempferol Aromadendrol (+)-Aromadendrin (+)-Dihydrokaempferol | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.213.374 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C15H12O6 | |
| Molar mass | 288.255 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Metabolism
The enzyme dihydrokaempferol 4-reductase uses cis-3,4-leucopelargonidin and NADP+ to produce (+)-aromadendrin, NADPH, and H+.
Glycosides
(2R,3R)-trans-Aromadendrin-7-O-beta-D-glucopyranoside-6′′-(4′′′-hydroxy-2′′′-methylene butanoate) is an acylated glucoside of aromadendrin isolated from the stem bark of Afzelia bella[2] (Fabaceae).
Phellamurin is the 8-prenyl 7-glucoside derivative of aromadendrin.
Chemistry
(+)-Leucopelargonidin, (2R,3S,4R)-3,4,5,7,4'-pentahydroxyflavan, can be synthesized from (+)-aromadendrin by sodium borohydride reduction.[3]
gollark: Arch Linux: Remove Windows in probably just 20 or more commands!
gollark: All you need are some nanometre-precision scissors and a very steady hand.
gollark: It's hard to make things which are good at *both* of those, and you would deal with twice the heat in one place.
gollark: CPUs have to execute x86 (or ARM or other things, but generally a documented, known instruction set) very fast sequentially, GPUs can execute basically whatever they want as long as it can be generated from one of the standard ways to interface with them, and do it in a massively parallel way.
gollark: It's not very efficient to have one thing do both because being specialized means they can make specific optimizations.
References
- V. I. Lutskii, A. S. Gromova and N. A. Tyukavkina (1971). "Aromadendrin, apigenin, and kaempferol from the wood of Pinus sibirica". Chemistry of Natural Compounds. 7 (2): 197. doi:10.1007/BF00568701.
- Binutu, OA; Cordell, GA (2001). "Constituents of Afzelia bella stem bark". Phytochemistry. 56 (8): 827–30. doi:10.1016/S0031-9422(01)00006-1. PMID 11324912.
- Heller, Werner; Britsch, Lothar; Forkmann, Gert; Grisebach, Hans (1985). "Leucoanthocyanidins as intermediates in anthocyanidin biosynthesis in flowers of Matthiola incana R. Br". Planta. 163 (2): 191. doi:10.1007/BF00393505. PMID 24249337.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.