Ammonium hexachloroiridate(IV)
Ammonium hexachloroiridate(IV) is the inorganic compound with the formula (NH4)2[IrCl6]. This dark brown solid is the ammonium salt of the iridium(IV) complex [IrCl6]2−. It is a commercially important iridium compound[1] one of the most common complexes of iridium(IV). A related but ill-defined compound is iridium tetrachloride, which is often used interchangeably.[2]
 | |
2PtCl6Xray.tif.png) | |
| Identifiers | |
|---|---|
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.037.264 |
| EC Number |
|
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| H8N2Cl6Ir | |
| Molar mass | 441.01 |
| Appearance | brown crystals |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
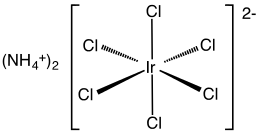
Structure
The compound has been characterized by X-ray crystallography. The salt crystallizes in a cubic motif like that of ammonium hexachloroplatinate. The [IrCl6]2− centers adopt octahedral molecular geometry.[3]
Uses
It is a key intermediate in the isolation of iridium from ores. Most other metals form insoluble sulfides when aqueous solutions of their chlorides are treated with hydrogen sulfide, but [IrCl6]2− resists ligand substitution. Upon heating under hydrogen, the solid salt converts to the metal:[1]
- (NH4)2[IrCl6] + 2 H2 → Ir + 6 HCl + 2 NH3
Bonding
The electronic structure of ammonium hexachloroiridate(IV) has attracted much attention. Its magnetic moment is less that than calculated for one electron. This result is interpreted as the result of antiferromagnetic coupling between Ir centers mediated by Cl---Cl interactions. Electron spin resonance, studies reveal that more than half of the spin density resides on chloride, thus the description of the complex as Ir(IV) is an oversimplification.[4]
References
- Renner, H.; Schlamp, G.; Kleinwächter, I.; Drost, E.; Lüschow, H. M.; Tews, P.; Panster, P.; Diehl, M.; et al. (2002). "Platinum group metals and compounds". Ullmann's Encyclopedia of Industrial Chemistry. Wiley. doi:10.1002/14356007.a21_075.
- Thomas R. B. Mitchell (2001). "Iridium(IV) Chloride". e-EROS Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.ri050.
- Bokii, G.B.; Ussikov, P.I. "Roentgenographische Untersuchung der Struktur des Ammonium-Chlor-Iridats (N H4)2IrCl6 Doklady Akademii Nauk SSSR 1940, vol. 26, p782-p784.
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. p. 1121. ISBN 978-0-08-037941-8.