Amino acid N-carboxyanhydride
Amino acid N-carboxyanhydrides, also called Leuchs' anhydrides, are reactive derivatives of amino acids. They are classified as N-carboxyanhydrides or NCAs. Typically these compounds are derived from amino acids by treatment with triphosgene. They are white solids, prone to polymerization upon treatment with nucleophiles.[1][2][3]
Preparation
NCA's were first synthesized in 1906 by Hermann Leuchs by heating an N-ethoxycarbonyl or N-methoxycarbonyl amino acid chloride in a vacuum at 50-70 °C.[4][5]
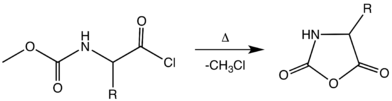
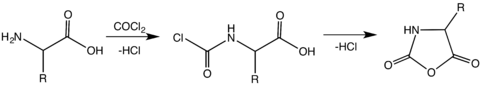
This synthesis of NCAs is sometimes called the Leuchs method. The relatively high temperatures necessary for this cyclization results in the decomposition of several NCAs. Of several improvements, one notable procedure involves treating an unprotected amino acid with phosgene or its trimer.[6][7][8]
Use in peptide synthesis
NCAs are used for peptide synthesis. Additionally, peptide synthesis reactions with NCAs does not require protection of the amino acid functional groups. NCA's are however highly reactive and their use can cogenerate many side products. N-substituted NCAs, such as sulfenamide derivatives, solve some of these problems.[9]
NCA's are precursors to amino acid homopolymers. Ephraim Katzir first used this method to synthesize poly-L-lysine from N-carbobenzyloxy-α-N-carboxy-L-lysine anhydride, followed by deprotection with phosphonium iodide.[10]
NCAs in siRNA delivery platform synthesis
One major drawback of siRNA-based therapies is that they lack sufficient in-vivo delivery of siRNA into the target cells because of the large size and negative charge density of siRNA. Efficient transfer of siRNA to the liver has been achieved by lipid nanoparticles, polymer conjugates, and peptide conjugates. One of the major challenges of current methods of transfer is the nonbiodegradability of the vessel once it is inside the body. This challenge is solved by using biodegradable poly(amide) polymers derived from polymerization of NCAs.[11]
Imine-NCA condensation
NCAs have been used for the synthesis of imidazolidinones, which are of interest in the pharmaceutical industry.[12]
See also
References
- Kricheldorf, H. R. (2006). "Polypeptides and 100 Years of Chemistry of Α-Amino Acid N-Carboxyanhydrides". Angewandte Chemie International Edition. 45 (35): 5752–5784. doi:10.1002/anie.200600693. PMID 16948174.
- Song, Z.; Han, Z.; Lv, S.; Chen, C.; Chen, L.; Yin, L.; Cheng, J. (2017). "Synthetic polypeptides: from polymer design to supramolecular assembly and biomedical application". Chem. Soc. Rev. 46: 6570–6599. doi:10.1039/C7CS00460E.CS1 maint: uses authors parameter (link)
- Kopecek, J. (2003). "Smart and genetically engineered biomaterials and drug delivery systems". Eur. J. Pharm. Sci. 20: 1–16. doi:10.1016/S0928-0987(03)00164-7.CS1 maint: uses authors parameter (link)
- Leuchs, Hermann (1906). "Ueber die Glycin-carbonsäure" [About the glycine-carboxylic acid] (PDF). Berichte der deutschen chemischen Gesellschaft (in German). 39: 857–61. doi:10.1002/cber.190603901133.
- Deming, T. J. (2007). "Synthetic polypeptides for biomedical applications". Prog. Polym. Sci. 32: 858–875. doi:10.1016/j.progpolymsci.2007.05.010.CS1 maint: uses authors parameter (link)
- Montalbetti, Christian A.G.N.; Falque, Virginie (2005). "Amide bond formation and peptide coupling". Tetrahedron. 61 (46): 10827–52. doi:10.1016/j.tet.2005.08.031.
- Lyndon C. Xavier, Julie J. Mohan, David J. Mathre, Andrew S. Thompson, James D. Carroll, Edward G. Corley, Richard Desmond (1997). "(S)-Tetrahydro-1-methyl-3,3-diphenyl-1h,3h-pyrrolo-[1,2-c][1,3,2]oxazaborole-borane Complex". Org. Synth. 74: 50. doi:10.15227/orgsyn.074.0050.CS1 maint: uses authors parameter (link)
- Matthew I. Gibson, Gregory J. Hunt, Neil R Cameron (2007). "Improved synthesis of O-linked, and first synthesis of S- linked, carbohydrate functionalised N-carboxyanhydrides (glycoNCAs)". Organic & Biomolecular Chemistry. 5 (17): 2756-2757. doi:10.1039/b707563d.CS1 maint: uses authors parameter (link)
- Katakai, Ryoichi (1975). "Peptide synthesis using o-nitrophenylsulfenyl N-carboxy α-amino acid anhydrides". The Journal of Organic Chemistry. 40 (19): 2697–2702. doi:10.1021/jo00907a001. PMID 1177065.
- Katchalski-Katzir, E. (2005). "My Contributions to Science and Society". Journal of Biological Chemistry. 280 (17): 16529–41. doi:10.1074/jbc.X400013200. PMID 15718236.
- Barrett, Stephanie E.; Burke, Rob S.; Abrams, Marc T.; Bason, Carol; Busuek, Marina; Carlini, Edward; Carr, Brian A.; Crocker, Louis S.; Fan, Haihong; Garbaccio, Robert M.; Guidry, Erin N.; Heo, Jun H.; Howell, Bonnie J.; Kemp, Eric A.; Kowtoniuk, Robert A.; Latham, Andrew H.; Leone, Anthony M.; Lyman, Michael; Parmar, Rubina G.; Patel, Mihir; Pechenov, Sergey Y.; Pei, Tao; Pudvah, Nicole T.; Raab, Conrad; Riley, Sean; Sepp-Lorenzino, Laura; Smith, Sheri; Soli, Eric D.; Staskiewicz, Steven; et al. (2014). "Development of a liver-targeted siRNA delivery platform with a broad therapeutic window utilizing biodegradable polypeptide-based polymer conjugates". Journal of Controlled Release. 183: 124–37. doi:10.1016/j.jconrel.2014.03.028. PMID 24657948.
- Sucu, Bilgesu Onur; Ocal, Nuket; Erden, Ihsan (2015). "Direct synthesis of imidazolidin-4-ones via cycloadditions of imines with a Leuchs' anyhdride". Tetrahedron Letters. 56 (20): 2590–2. doi:10.1016/j.tetlet.2015.04.002.