Alpha and beta carbon
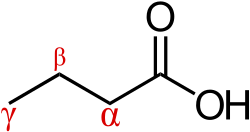
The alpha carbon (Cα) in organic molecules refers to the first carbon atom that attaches to a functional group, such as a carbonyl. The second carbon atom is called the beta carbon (Cβ), and the system continues naming in alphabetical order with Greek letters.
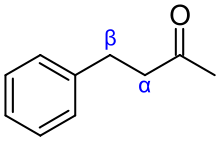
The nomenclature can also be applied to the hydrogen atoms attached to the carbon atoms. A hydrogen atom attached to an alpha carbon atom is called an alpha-hydrogen atom, a hydrogen atom on the beta-carbon atom is a beta hydrogen atom, and so on.
This naming standard may not be in compliance with IUPAC nomenclature, which encourages that carbons be identified by number, not by Greek letter, but it nonetheless remains very popular, in particular because it is useful in identifying the relative location of carbon atoms to other functional groups.
Organic molecules with more than one functional group can be a source of confusion. Generally the functional group responsible for the name or type of the molecule is the 'reference' group for purposes of carbon-atom naming. For example, the molecules nitrostyrene and phenethylamine are very similar; the former can even be reduced into the latter. However, nitrostyrene's α-carbon atom is adjacent to the phenyl group; in phenethylamine this same carbon atom is the β-carbon atom, as phenethylamine (being an amine rather than a styrene) counts its atoms from the opposite "end" of the molecule.[1]
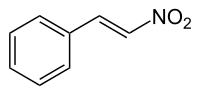 Nitrostyrene
Nitrostyrene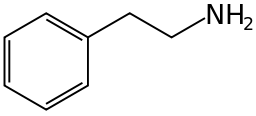 Phenethylamine
Phenethylamine
Examples
Proteins and amino acids
Alpha-carbon (α-carbon) is also a term that applies to proteins and amino acids. It is the backbone carbon before the carbonyl carbon atom in the molecule. Therefore, reading along the backbone of a typical protein would give a sequence of –[N—Cα—carbonyl C]n– etc. (when reading in the N to C direction). The α-carbon is where the different substituents attach to each different amino acid. That is, the groups hanging off the chain at the α-carbon are what give amino acids their diversity. These groups give the α-carbon its stereogenic properties for every amino acid except for glycine. Therefore, the α-carbon is a stereocenter for every amino acid except glycine. Glycine also does not have a β-carbon, while every other amino acid does.
The α-carbon of an amino acid is significant in protein folding. When describing a protein, which is a chain of amino acids, one often approximates the location of each amino acid as the location of its α-carbon. In general, α-carbons of adjacent amino acids in a protein are about 3.8 ångströms (380 picometers) apart.
Enols and enolates
The α-carbon is important for enol- and enolate-based carbonyl chemistry as well. Chemical transformations affected by the conversion to either an enolate or an enol, in general, lead to the α-carbon acting as a nucleophile, becoming, for example, alkylated in the presence of primary haloalkane. An exception is in reaction with silyl chlorides, bromides, and iodides, where the oxygen acts as the nucleophile to produce silyl enol ether.
References
- "Nomenclature". Ask Dr. Shulgin Online. Retrieved August 5, 2010.
External links
