Alkyne metathesis
Alkyne metathesis is an organic reaction that entails the redistribution of alkyne chemical bonds. The reaction requires metal catalysts. Mechanistic studies show that the conversion proceeds via the intermediacy of metal alkylidyne complexes.[1] The reaction is related to olefin metathesis.

History
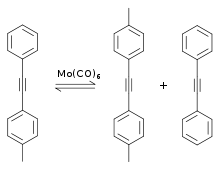
Metal-catalyzed alkyne metathesis was first described in 1968 by Bailey, et al. The Bailey system utilized a mixture of tungsten and silicon oxides at temperatures as high as 450 °C. In 1974 Mortreux reported the use of a homogeneous catalyst—molybdenum hexacarbonyl at 160 °C—to observe an alkyne scrambling phenomenon, in which an unsymmetrical alkyne equilibrates with its two symmetrical derivatives.[2] The Mortreux system consists of the molybdenum precatalyst molybdenum hexacarbonyl Mo(CO)6 and resorcinol cocatalyst. In 1975 T.J. Katz proposed a metal carbyne (i.e. alkylidyne) and a metallacyclobutadiene as intermediates. In 1981 R.R. Schrock characterized several metallacyclobutadiene complexes that were catalytically active. [3]
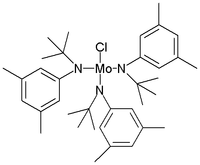
In 2001, Alois Fürstner reported a new molybdenum catalyst replacing alkoxide with aniline-derived ligands.[4]
Catalysts
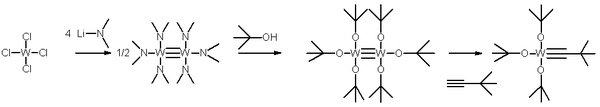
The Schrock catalyst is tris(t-butoxy)(2,2-dimethylpropylidyne)tungsten(VI). Commercially available, it is prepared from W2(NMe2)6, which undergoes alcoholysis with tert-butanol.
Ring closing alkyne metathesis
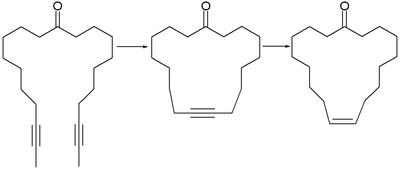
Alkyne metathesis can be used in ring-closing operations and RCAM stands for ring closing alkyne metathesis. The olfactory molecule civetone can be synthesised from a di-alkyne. After ring closure the new triple bond is stereoselectively reduced with hydrogen and the Lindlar catalyst in order to obtain the Z-alkene (cyclic E-alkenes are available through the Birch reduction). An important driving force for this type of reaction is the expulsion of small gaseous molecules such as acetylene or 2-butyne.
The same two-step procedure was used in the synthesis of the naturally occurring cyclophane turriane.
Trisamidomolybdenum(VI) alkylidyne complexes catalyze alkyne metathesis.[5]
Nitrile-alkyne cross-metathesis
By replacing a tungsten alkylidyne by a tungsten nitride and introducing a nitrile Nitrile-Alkyne Cross-Metathesis or NACM couples two nitrile groups together to a new alkyne. Nitrogen is collected by use of a sacrificial alkyne (elemental N2 is not formed):[6][7]
References
- Fürstner, A.; Davies, P. W. (2005). "Alkyne Metathesis". Chemical Communications (18): 2307–2320. doi:10.1039/b419143a. PMID 15877114. S2CID 40674318.
- Fürstner, A.; Mathes, C.; Lehmann, C. W. (1999). "Mo[N(t-Bu)(Ar)]3 Complexes As Catalyst Precursors: In Situ Activation and Application to Metathesis Reactions of Alkynes and Diynes". J. Am. Chem. Soc. 121 (40): 9453–9454. doi:10.1021/ja991340r. hdl:11858/00-001M-0000-0024-1DF5-F.
- Schrock, R. R.; Clark, D. N.; Sancho, J.; Wengrovius, J. H.; Rocklage, S. M.; Pedersen, S. F. (1982). "Tungsten(VI) neopentylidyne complexes". Organometallics. 1 (12): 1645–1651. doi:10.1021/om00072a018.
- Mortreux, Andre (1974). "Metathesis of alkynes by a molybdenum hexacarbonyl–resorcinol catalyst". Chemical Communications (19): 786–787. doi:10.1039/C39740000786.
- Wei Zhang, Yunyi Lu, Jeffrey S. Moore (2007). "Preparation of a Trisamidomolybdenum(VI) Propylidyne Complex". Org. Synth. 84: 163. doi:10.15227/orgsyn.084.0163.CS1 maint: uses authors parameter (link)Wei Zhang, Hyeon Mo Cho, Jeffrey S. Moore (2007). "Preparation of a Carbazole-Based Macrocycle via Precipitation-driven Alkyne Metathesis". Org. Synth. 84: 177. doi:10.15227/orgsyn.084.0177. S2CID 93992722.CS1 maint: uses authors parameter (link)
- Geyer, A. M.; Gdula, R. K.; Wiedner, E. S.; Johnson, M. J. A. (2007). "Catalytic Nitrile-Alkyne Cross-Metathesis". J. Am. Chem. Soc. 129 (13): 3800–3801. doi:10.1021/ja0693439. PMID 17355136.
- Ritter, S. (March 26, 2007). "Nitrile-Alkyne Cross-Metathesis". Chemical & Engineering News.