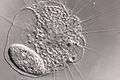
Actinophryid
The actinophryids are an order of heliozoa. They are the most common heliozoa in fresh water and can also be found in marine and soil habitats. Actinophryids are unicellular and roughly spherical in shape, with many axopodia that radiate outward from the cell body. Axopodia are a type of pseudopodia that are supported by hundreds of microtubules arranged in a needle-like internal structure. These axopods adhere to passing prey and assist with cell movement, as well as playing a part in cell division and cell fusion.[1][2]
| Actinophryid | |
|---|---|
 | |
| Scientific classification | |
| Kingdom: | Chromista |
| Phylum: | Ochrophyta |
| Class: | Actinochrysophyceae |
| Order: | Actinophryida Hartmann 1913 |
| Genera | |
|
Actinophrys | |
Description
Actinophryids are largely aquatic protozoa with a spherical cell body and many needle-like axopodia. They resemble the shape of a sun due to this structure, which is the inspiration for their common name: heliozoa, or "sun-animalcules". They range in size from a few micrometers to a full millimeter across.[3]
The cell body is largely vacuolated, with the ectoplasm consisting almost entirely of these structures. The endoplasm of actinophryids is often darker and denser than the outer layer, and can sometimes be seen as a sharp boundary under a light microscope.[4] The organisms can be either mononucleate, with a single, well defined nucleus in the center of the cell body, or multinucleate, with 10 or more nuclei dispersed throughout the organism. The cytoplasm of actinophryids is often granular, similar to that of Amoeba.[5]
Contractile vacuoles are common in these organisms, who use them to maintain homeostasis and control buoyancy. These are visible as clear bulges from the surface of the cell body that slowly fill then rapidly deflate, expelling the contents into the environment.
Axopodia

The most distinctive characteristic of the actinophryids is their axopodia. These axopodia consist of a central, rigid rod which is coated in a thin layer of ectoplasm. These axonemes are rooted in the endoplasm and terminate there, sometimes close to a nucleus.[5] The axonemes are composed microtubules arranged in a double spiral pattern characteristic of the order.[6] Due to their long, parallel construction these microtubules demonstrate strong birefringence.[7][8]
These axopodia are used for prey capture, mobility, and cell fusion and division.[1][2] They can be flexible, especially when the organisms are starved,[4] and are highly dynamic, undergoing frequent construction and destruction. When used to collect prey items, two methods of capture have been noted, termed axopodial flow and rapid axopodial contraction.[1] Axopodial flow involves the slow movement of a prey item along the surface of the axopod as the ectoplasm itself moves, while rapid axopodial contraction involves the collapse of the axoneme's microtubule structure.[8] This behavior has been documented in many species, including Actinosphaerium nucleofilum, Actinophrys sol, and Raphidiophrys contractilis.[8][9][10] The rapid axopodial contraction occurs at high speed, often in excess of 5mm/s or tens of body lengths per second.[11]
The axopodial contractions have been shown to be highly sensitive to environmental factors such as temperature and pressure[7][12] as well as chemical signals like Ca2+ and colchicine.[9][13][14] They may also be triggered by mechanical or electrical stimulation.[2][11]
Reproduction
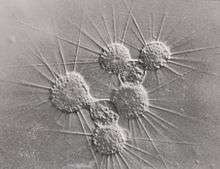
Reproduction in actinophryids generally takes place via fission, where one parent cell divides into two or more daughter cells. For multinucleate heliozoa, this process is plasmotomic as the nuclei are not duplicated prior to division.[4] It has been observed that reproduction appears to be a response to food scarcity, with an increased number of divisions following the removal of food and larger organisms during times of food excess.[15]
Actinophryids also undergo autogamy during times of food scarcity. This is better described as genetic reorganization than reproduction, as the number of individuals produced is the same as the initial number. Nonetheless, it serves as a way to increase genetic diversity within an individual which may improve the likelihood of expressing favorable genetic traits.[16]
Plastogamy has also been extensively documented in actinophryids, especially in multinucleate ones. Actinosphaerium were observed to combine freely without the combination of nuclei, and this process sometimes resulted in more or less individuals than originally combined. This process is not caused merely by contact between two individuals but can be caused by damage to the cell body.[15]
Cyst function and formation
Under unfavourable conditions, some species will form a cyst. This is often the product of autogamy, in which case the cysts produced are zygotes.[16] Cells undergoing this process withdraw their axopodia, adhere to the substrate, and take on an opaque and grayish appearance.[17] This cyst then divides until only uninucleate cells remain. The cyst wall is thickly layered 7-8 times and includes gelatinous layers, layers of silica plates, and iron.[18]
Taxonomy
Originally placed in Heliozoa (Sarcodina), the group's current location within the larger tree of life is debated. It may belong to either the Actinochrysophyceae (Axodines), or to Raphidomonadea.
There are several genera included within this classification. Actinophrys are smaller and have a single, central nucleus.[9] Most have a cell body 40-50 micrometer in diameter with axopods around 100 μm in length, though this varies significantly. Actinosphaerium are several times larger, from 200-1000 μm in diameter, with many nuclei[9] and are found exclusively in fresh water.[19] A third genus, Camptonema, was named as a junior subjective synonym of Actinosphaerium by Mikrjukov & Patterson in 2001,[20] but Cavalier-Smith & Scoble (2013) preserve the genus.[3] Heliorapha was also added to this classification by Cavalier & Smith (2013), which was previously the genus Ciliophrys.[3]
Classification based on Cavalier-Smith and Scoble 2013[3][21]
- In class Raphidomonadea Silva 1980 emend. Cavalier-Smith 2013 [Raphidophyceae Chadefaud 1950 emend. Silva 1980 s.l.]
- In subclass Raphopoda Cavalier-Smith 2013
- Order Actinophyrida Hartmann 1913 [Actinophrydia Kühn 1926; Actinophrydea Hartmann 1913]
- Family Actinosphaeriidae Cavalier-Smith 2013
- Genus Actinosphaerium Ritter von Stein 1857 [Echinosphaerium Hovasse 1965]
- Genus Camptonema Schaudinn 1894
- Family Helioraphidae Cavalier-Smith 2013
- Genus Heliorapha Cavalier-Smith 2013
- Family Actinophryidae Dujardin 1841
- Genus Actinophrys Ehrenberg 1830 [Trichoda Müller 1773 nomen oblitum; Peritricha Bory de St.Vincent 1824 nomen dubium non Stein 1859]
- Family Actinosphaeriidae Cavalier-Smith 2013
- Order Actinophyrida Hartmann 1913 [Actinophrydia Kühn 1926; Actinophrydea Hartmann 1913]
- In subclass Raphopoda Cavalier-Smith 2013
Gallery
References
- Suzaki, T.; Shigenaka, Y.; Watanabe, S.; Toyohara, A. (1980). "Food capture and ingestion in the large heliozoan, Echinosphaerium nucleofilum". Journal of Cell Science. 42: 61–79. ISSN 0021-9533. PMID 7400244.
- Ando, Motonori; Shigenaka, Yoshinobu (1989). "Structure and function of the cytoskeleton in heliozoa: I. Mechanism of rapid axopodial contraction in Echinosphaerium". Cell Motility and the Cytoskeleton. 14 (2): 288–301. doi:10.1002/cm.970140214.
- Cavalier-Smith, T; Scoble, JM (August 2013). "Phylogeny of Heterokonta: Incisomonas marina, a uniciliate gliding opalozoan related to Solenicola (Nanomonadea), and evidence that Actinophryida evolved from raphidophytes". European Journal of Protistology. 49 (3): 328–53. doi:10.1016/j.ejop.2012.09.002. PMID 23219323.
- Barrett, J. (August 1958). "Some Observations on Actinosphaerium nucleofilum n. sp., a New Fresh Water Actinophryid". The Journal of Protozoology. 5 (3): 205–209. doi:10.1111/j.1550-7408.1958.tb02553.x.
- Anderson, E.; Beams, H. W. (May 1960). "The Fine Structure of the Heliozoan, Actinosphaerium nucleofilum". The Journal of Protozoology. 7 (2): 190–199. doi:10.1111/j.1550-7408.1960.tb00729.x.
- Gast, R.J. (2017). "Centrohelida and Other Heliozoan-like Protists". In Archibald, J.; Simpson, A.; Slamovits, C.; Margulis, L.; Melkonian, M.; Chapman, D.; Corliss, J. (eds.). Handbook of the Protists. Cham, Switzerland: Springer International. pp. 1–17. doi:10.1007/978-3-319-32669-6_28-1. ISBN 978-3-319-32669-6.
- Tilney, L.; Porter, K. (July 1967). "Studies on the microtubules in heliozoa II. The effect of low temperature on these structures in the formation and maintenance of the axopodia". Journal of Cell Biology. 34 (1): 327–343. doi:10.1083/jcb.34.1.327. PMC 2107222. PMID 6033539.
- Suzaki, Toshinobu; Ando, Motonori; Inai, Yoko; Shigenaka, Yoshinobu (November 1994). "Structure and function of the cytoskeleton in heliozoa". European Journal of Protistology. 30 (4): 404–413. doi:10.1016/S0932-4739(11)80215-4.
- Kinoshita, E; Shigenaka, Y; Suzaki, T (2001). "The ultrastructure of contractile tubules in the heliozoon Actinophrys sol and their possible involvement in rapid axopodial contraction". The Journal of Eukaryotic Microbiology. 48 (5): 519–26. doi:10.1111/j.1550-7408.2001.tb00187.x. PMID 11596916.
- KINOSHITA, EIJI; SUZAKI, TOSHINOBU; SHIGENAKA, YOSHINOBU; SUGIYAMA, MASANORI (May 1995). "Ultrastructure and Rapid Axopodial Contraction of a Heliozoa, Raphidiophrys contractilis Sp. Nov". The Journal of Eukaryotic Microbiology. 42 (3): 283–288. doi:10.1111/j.1550-7408.1995.tb01581.x.
- Shigenaka, Y.; Yano, K.; Suzaki, T. (1982). "Shigenaka, Y., Yano, K., Yogosawa, R. and Suzaki, T., 1982. Rapid contraction of the microtubule-containing axopodia in a large heliozoan Echinosphaerium". Biological functions of microtubules and related structures. Tokyo: Academic Press. pp. 105–114.
- Tilney, Lewis G.; Byers, Breck (1 October 1969). "Studies on the Microtubules in Heliozoa V. Factors Controlling the Organization of Microtubules in the Axonemal Pattern in Echinosphaerium nucleofilum". The Journal of Cell Biology. 43 (1): 148–165. doi:10.1083/jcb.43.1.148. ISSN 0021-9525. PMC 2107851. PMID 5824062.
- Tilney, L. (December 1968). "Studies on the microtubules in heliozoa. IV. The effect of colchicine on the formation and maintenance of the axopodia and the redevelopment of pattern in Actinosphaerium nucleofilum (Barrett)". Journal of Cell Science. 3 (4): 549–62. PMID 5707852.
- Khan, SM; Arikawa, M; Omura, G; Suetomo, Y; Kakuta, S; Suzaki, T (November 2003). "Axopodial contraction in the heliozoon Raphidiophrys contractilis requires extracellular Ca2+". Zoological Science (Submitted manuscript). 20 (11): 1367–72. doi:10.2108/zsj.20.1367. PMID 14624035.
- Johnson, Herbert P. (April 1894). "The plastogamy of actinosphaerium". Journal of Morphology. 9 (2): 269–276. doi:10.1002/jmor.1050090206. hdl:2027/hvd.32044107306375.
- Grell, Karl Gottlieb (2013). Protozoology. Berlin, Heidelberg: Springer Berlin Heidelberg. pp. 178–181. ISBN 9783642619588.
- MacKinnon, D. L. (1906). "A few Observations on the Encystation of Actinosphaerium eichhorni under different conditions of Temperature" (PDF). Quarterly Journal of Microscopical Science. 52: 407–422. Retrieved 2 January 2018.
- Patterson, D.; Thompson, D. (May 1981). "Structure and Elemental Composition of the Cyst Wall of Echinosphaerium nucleofilum Barrett (Heliozoea, Actinophryida)". The Journal of Protozoology. 28 (2): 188–192. doi:10.1111/j.1550-7408.1981.tb02831.x.
- "Actinosphaerium eichhornii". Microworld. 2019-02-28. Retrieved 2020-01-29.
- A. MIKRJUKOV, Kirill; J. PATTERSON, David (1 February 2001). "Taxonomy and Phylogeny of Heliozoa. III. Actinophryids". Acta Protozoologica. 40: 3–25.
- Guiry, M.D.; Guiry, G.M. (2016). "Raphidophyceae". AlgaeBase (3). Retrieved 2016-08-26.
| Wikispecies has information related to Actinophryida |