Acetonitrile (data page)
This page provides supplementary chemical data on acetonitrile.
Material Safety Data Sheet
The handling of this chemical may incur notable safety precautions. It is highly recommend that you seek the Material Safety Datasheet (MSDS) for this chemical from a reliable source and follow its directions.
Structure and properties
| Structure and properties | |
|---|---|
| Index of refraction, nD | 1.344 at 20.0°C [1] |
| Abbe number | ? |
| Dielectric constant, εr | 36.64 ε0 at 20 °C [1] |
| Dipole Moment, | 3.84 D |
| Bond strength | ? |
| Bond length | ? |
| Bond angle | ? |
| Magnetic susceptibility | ? |
| Viscosity[2] | 0.441 mPa·s at 0°C 0.343 mPa·s at 25°C |
| Surface tension[2] | 29.29 dyn/cm |
Thermodynamic properties
| Phase behavior | |
|---|---|
| Triple point[3] | 229.32 K (−43.83 °C), 167 Pa |
| Critical point | 545 K (272 °C), 4.87 MPa |
| Std enthalpy change of fusion, ΔfusH |
8.167 kJ/mol (crystal I → liq) |
| Std entropy change of fusion, ΔfusS |
35.61 J/(mol·K) (crystal I → liq) |
| Std enthalpy change of vaporization, ΔvapH |
33.225 kJ/mol at 25°C 29.75 at 81.6°C (BP) |
| Std entropy change of vaporization, ΔvapS |
111.44 J/(mol·K) at 25°C |
| Solid properties | |
| Std enthalpy change of formation, ΔfH |
? kJ/mol at 25°C |
| Standard molar entropy, S |
? J/(mol K) |
| Heat capacity, cp | 92.36 J/(mol K)at 298.15 K |
| Std enthalpy change of state transition, ΔtrsH |
0.8979 kJ/mol at −56.2 °C (crystal II → crystal I) |
| Std entropy change of state transition, ΔtrsS |
4.14 J/(mol·K) at −56.2 °C (crystal II → crystal I) |
| Liquid properties | |
| Std enthalpy change of formation, ΔfH |
−40.56 kJ/mol |
| Standard molar entropy, S |
149.62 J/(mol K) |
| Enthalpy of combustion, ΔcH |
−1256.33 kJ/mol |
| Heat capacity, cp | 91.7 J/(mol K) at 25°C |
| Gas properties | |
| Std enthalpy change of formation, ΔfH |
−74.04 kJ/mol |
| Standard molar entropy, S |
? J/(mol K) |
| Heat capacity, cp | ? J/(mol K) |
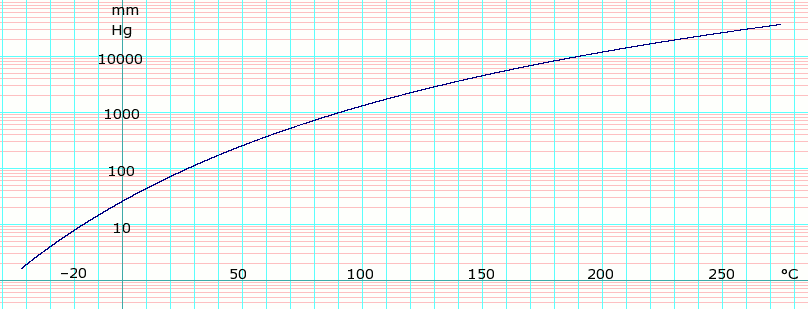
Vapor pressure of liquid
| P in mm Hg | 1 | 10 | 40 | 100 | 400 | 760 | |
| T in °C | −47.0(s) | −16.3 | 7.7 | 27.0 | 62.5 | 81.8 | |
Table data obtained from CRC Handbook of Chemistry and Physics, 44th ed. The "(s)" notation indicates temperature of solid/vapor equilibrium. Otherwise the data is temperature of liquid/vapor equilibrium.
Distillation data
|
|
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Spectral data
| UV-Vis | |||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| λmax | ? nm | ||||||||||||||||||||||||||||||||||||
| Extinction coefficient, ε | ? | ||||||||||||||||||||||||||||||||||||
| IR | |||||||||||||||||||||||||||||||||||||
| Major absorption bands[5] |
| ||||||||||||||||||||||||||||||||||||
| NMR | |||||||||||||||||||||||||||||||||||||
| Proton NMR | |||||||||||||||||||||||||||||||||||||
| Carbon-13 NMR | |||||||||||||||||||||||||||||||||||||
| Other NMR data | |||||||||||||||||||||||||||||||||||||
| MS | |||||||||||||||||||||||||||||||||||||
| Masses of main fragments |
|||||||||||||||||||||||||||||||||||||
gollark: This is somewhat problematic.
gollark: I see.
gollark: > ALL PASSING IS BY REFERENCE.So if I want to pass a bool, it will actually be an 8-byte pointer?
gollark: I mean, I feel like Rust makes it pretty clear.
gollark: No gotchas.
References
- "Sigma Aldrich Solvent Center". Retrieved 22 May 2014.
- "Pure Component Properties" (Queriable database). Chemical Engineering Research Information Center. Archived from the original on 3 June 2007. Retrieved 15 May 2007.
- Vapor Pressures of Acetonitrile Determined by Comparative Ebulliometry, Michael B. Ewing* and Jesus C. Sanchez Ochoa, Journal of Chemical & Engineering Data 2004 49 (3), 486-491
- "Binary Vapor-Liquid Equilibrium Data" (Queriable database). Chemical Engineering Research Information Center. Retrieved 15 May 2007.
- "Spectral Database for Organic Compounds". Advanced Industrial Science and Technology. Archived from the original (Queriable database) on 5 May 2006. Retrieved 7 June 2007.
- "NIST Standard Reference Database". Archived from the original on 23 May 2007. Retrieved 15 May 2007.
Except where noted otherwise, data relate to standard ambient temperature and pressure.
Disclaimer applies.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.