Acetone imine
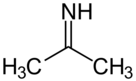
Acetone imine, or 2-propanimine is an organic compound and an imine with the chemical formula (CH3)2CNH. It is a volatile and flammable liquid at room temperature. It is the simplest ketimine. This compound is mainly of academic interest.
| |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
2-Propanimine | |||
| Systematic IUPAC name
Propan-2-imine[1] | |||
Other names
| |||
| Identifiers | |||
| ChemSpider | |||
| MeSH | Imine Acetone Imine | ||
PubChem CID |
|||
CompTox Dashboard (EPA) |
|||
| Properties | |||
| C3H7N | |||
| Molar mass | 57.096 g·mol−1 | ||
| Appearance | colorless liquid | ||
| Density | 0.8 g cm−3 (25 °C) | ||
| Boiling point | 57–59 °C (135–138 °F; 330–332 K) | ||
| log P | -0.56 | ||
Refractive index (nD) |
1.394 | ||
| Hazards | |||
| GHS pictograms |   | ||
| GHS Signal word | Danger | ||
GHS hazard statements |
H225, H319, H336 | ||
| P210, P261, P305+351+338 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 14.7 °C (58.5 °F; 287.8 K) | ||
| Related compounds | |||
Related compounds |
Acetone oxime | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Synthesis and reactions
Acetone imine is prepared by dehydrocyanation of the cyanoamine of acetone, which is prepared from acetone cyanohydrin. Dicyclohexylcarbodiimide (CyN=C=NCy) serves as the scavenger for hydrogen cyanide:[2]
- (CH3)2C(NH2)CN + CyN=C=NCy → (CH3)2CNH + CyN(H)-C(CN)=NCy
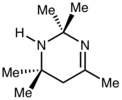 Upon standing at room temperature, samples of acetone imine degrade to give this heterocycle, called acetonin.
Upon standing at room temperature, samples of acetone imine degrade to give this heterocycle, called acetonin.
The compound hydrolyzes readily:
- (CH3)2CNH + H2O → (CH3)2CO + NH3
This reactivity is characteristic of imines derived from ammonia. Methylene imine (CH2=NH) is also highly reactive, condensing to hexamethylenetetramine. Upon standing, the imine undergoes further condensation to give the tetrahydropyrimidine called acetonin, with loss of ammonia.[3]
The imine of hexafluoroacetone, ((CF3)2C=NH) is by contrast robust.[4]
References
- "Synonyms". Pubchem.
- K. Findeisen; H. Heitzer; K. Dehnicke (1981). "Neue Methode zur Herstellung von Aldiminen und Ketiminen". Synthesis: 702–704. doi:10.1055/s-1981-29566.
- Matter, E. (1947). "Über ein neues Reaktionsprodukt aus Aceton und Ammoniak (Acetonin) (A new reaction product from acetone and ammonia (acetonine)) I". Helvetica Chimica Acta. 30: 1114–23. doi:10.1002/hlca.19470300503.
- W. J. Middleton, H. D. Carlson (1970). "Hexafluoroacetone imine". Org. Syntheses. 50: 81–3. doi:10.15227/orgsyn.050.0081..