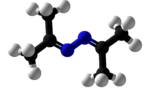
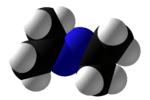
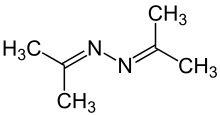
Acetone azine
Acetone azine is the simplest ketazine. It is an intermediate in some hydrazine manufacturing processes.
 | |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
1,2-di(propan-2-ylidene)hydrazine | |||
| Systematic IUPAC name
Acetone azine | |||
| Other names
Ketazine Acetone ketazine | |||
| Identifiers | |||
3D model (JSmol) |
|||
| 4-01-00-03207 | |||
| ChemSpider | |||
| ECHA InfoCard | 100.010.009 | ||
| EC Number |
| ||
PubChem CID |
|||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C6H12N2 | |||
| Molar mass | 112.17 g mol−1 | ||
| Appearance | Pale-yellow liquid | ||
| Density | 0.842 g cm−3 | ||
| Melting point | −125 °C (−193 °F; 148 K) | ||
| Boiling point | 133 °C (271 °F; 406 K) | ||
Refractive index (nD) |
1.454 | ||
| Hazards | |||
| GHS pictograms |    | ||
| GHS Signal word | Danger | ||
GHS hazard statements |
H226, H302, H311, H315, H319, H335, H350 | ||
| P201, P261, P280, P305+351+338, P308+313 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 31 °C (88 °F; 304 K) | ||
| Related compounds | |||
Related compounds |
Hydrazine | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Synthesis
Acetone azine can be prepared from acetone and hydrazine:[3]
- 2 (CH3)2CO + N2H4 → 2 H2O + [(CH3)2C=N]2
It can also be produced from acetone (2 eq.), ammonia (2 eq.) and hydrogen peroxide (1 eq.).[4] The first step is the formation of acetone imine, Me2C=NH; this is then oxidized by hydrogen peroxide through a complex mechanism to give 3,3-dimethyloxaziridine, which reacts with a further molecule of ammonia to produce acetone hydrazone. The hydrazone then condenses with a further molecule of acetone to produce the azine. The acetone azine product is distilled out of the reaction mixture as its azeotrope with water (n(H2O)/n(azine) ≈ 6).[5]
Reactions
Acetone azine can be used to prepare acetone hydrazone[3] and 2-diazopropane.[6]
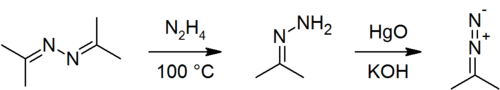
Hydrazine can be produced through acid-catalysed hydrolysis of acetone azine:[7]
- 2 H2O + [(CH3)2C=N]2 → 2 (CH3)2CO + N2H4
References
- "Acetone azine MSDS (Santa Cruz Biotechnology)" (PDF).
- "Acetone azine MSDS (Sigma Aldrich)".
- Day, A. C.; Whiting, M. C. "Acetone Hydrazone". Organic Syntheses.; Collective Volume, 6, p. 10
- US 3972878, Schirmann, Jean-Pierre; Jean Combroux & Serge Yvon Delavarenne, "Method for preparing azines and hydrazones", issued 1976-08-03, assigned to Produits Chimiques Ugine Kuhlmann.US 3978049, Schirmann, Jean-Pierre; Pierre Tellier & Henri Mathais et al., "Process for the preparation of hydrazine compounds", issued 1976-08-31, assigned to Produits Chimiques Ugine Kuhlmann
- US 4724133, Schirmann, Jean-Pierre; Jean Combroux & Serge Y. Delavarenne, "Preparation of a concentrated aqueous solution of hydrazine hydrate", issued 1988-02-09, assigned to Atochem
- Organic Syntheses, Coll. Vol. 6, p.392 (1988); Vol. 50, p.27 (1970). Link
- Gilbert, E. C. (1929), "Studies on Hydrazine. The Hydrolysis of Dimethylketazine and the Equilibrium between Hydrazine and Acetone", J. Am. Chem. Soc., 51 (11): 3394–3409, doi:10.1021/ja01386a032.