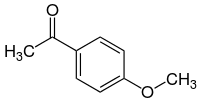
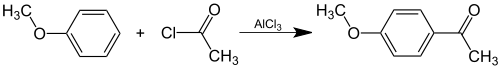Acetanisole
Acetanisole is an aromatic chemical compound with an aroma described as sweet, fruity, nutty, and similar to vanilla. In addition Acetanisole can sometimes smell like butter or caramel. [3]
 | |
| Names | |
|---|---|
| Preferred IUPAC name
1-(4-Methoxyphenyl)ethan-1-one | |
| Other names
4-Acetylanisole; para-Acetanisole; 4-Methoxyacetophenone; Linarodin; Novatone; Vananote; Castoreum anisole; 4-Methoxyphenyl methyl ketone | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.002.560 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C9H10O2 | |
| Molar mass | 150.177 g·mol−1 |
| Appearance | White to pale yellow crystals[1] |
| Density | 1.094 g/cm3 |
| Melting point | 38.5 °C (101.3 °F; 311.6 K)[2] |
| Boiling point | 258 °C (496 °F; 531 K)[2] |
| 2470 mg/L[2] | |
| Hazards | |
| Flash point | 138 °C (280 °F)[3] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Acetanisole is found naturally in castoreum, the glandular secretion of the beaver.[1]
Preparation
Acetanisole can be prepared synthetically by Friedel-Crafts acylation of anisole with acetyl chloride:
Application
It is used as a cigarette additive,[4] a fragrance,[1] and a flavoring in food.[5]
- In the pharmaceutical sector, acetanisole is used in the synthesis of Benfurodil hemisuccinate.
- It can also be used to synthesize basic stimulant compounds such as methyl-synephrine and methyl-hordenine, which were detected in the energy supplement Meltdown®.[6]
- In the case of m-acetanisole, this regioisomer was used to synthesize oxyfedrine.
- Sulfarlem (Anethole trithione) for treating Parkinson's disease.
Appearance
At room temperature 4-Methoxyacetophenone is solid, and has a white crystal like structure. Once melted, the white crystals turn into a clear liquid.
gollark: A Linux-based one would allow me to avoid the very hard work of "implementing every network driver ever".
gollark: I mean, that would be cooler but involve a lot of duplicated effort and complexity.
gollark: Oh, and a CC program to connect to that and run commands like "update" and "full restart" and "configure networking".
gollark: If I were to ever get round to implementing this, it would use Alpine or something similar, and just ship with CraftOS-PC automatically started on boot, as well as a websocket-accessible daemon to let it run commands on the real device.
gollark: Very "useful".
References
- Para-Acetanisole, The Good Scents Company
- Acetanisole in the ChemIDplus database
- Acetanisole at Sigma-Aldrich
- Tobacco Documents | Profiles | Additives | Acetanisole Archived April 11, 2008, at the Wayback Machine
- 21 C.F.R. 172.515
- Hoffman, Jay R; Kang, Jie; Ratamess, Nicholas A; Rashti, Stefanie L; Tranchina, Christopher P; Faigenbaum, Avery D (2009). "Thermogenic effect of an acute ingestion of a weight loss supplement". Journal of the International Society of Sports Nutrition. 6 (1): 1. doi:10.1186/1550-2783-6-1. ISSN 1550-2783. PMC 3313118.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.