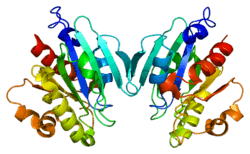ARF5
ADP-ribosylation factor 5 is a protein that in humans is encoded by the ARF5 gene.[5][6]
ADP-ribosylation factor 5 (ARF5) is a member of the human ARF gene family. These genes encode small guanine nucleotide-binding proteins that stimulate the ADP-ribosyltransferase activity of cholera toxin and play a role in vesicular trafficking and as activators of phospholipase D. The gene products include 6 ARF proteins and 11 ARF-like proteins and constitute 1 family of the RAS superfamily. The ARF proteins are categorized as class I (ARF1, ARF2, and ARF3), class II (ARF4 and ARF5) and class III (ARF6). The members of each class share a common gene organization. The ARF5 gene spans approximately 3.2kb of genomic DNA and contains six exons and five introns.[6]
Interactions
ARF5 has been shown to interact with ARFIP2.[7][8]
References
- GRCh38: Ensembl release 89: ENSG00000004059 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000020440 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- Tsuchiya M, Price SR, Tsai SC, Moss J, Vaughan M (March 1991). "Molecular identification of ADP-ribosylation factor mRNAs and their expression in mammalian cells". J Biol Chem. 266 (5): 2772–7. PMID 1993656.
- "Entrez Gene: ARF5 ADP-ribosylation factor 5".
- Kanoh, H; Williger B T; Exton J H (February 1997). "Arfaptin 1, a putative cytosolic target protein of ADP-ribosylation factor, is recruited to Golgi membranes". J. Biol. Chem. UNITED STATES. 272 (9): 5421–9. doi:10.1074/jbc.272.9.5421. ISSN 0021-9258. PMID 9038142.
- Shin, O H; Exton J H (August 2001). "Differential binding of arfaptin 2/POR1 to ADP-ribosylation factors and Rac1". Biochem. Biophys. Res. Commun. United States. 285 (5): 1267–73. doi:10.1006/bbrc.2001.5330. ISSN 0006-291X. PMID 11478794.
External links
- Human ARF5 genome location and ARF5 gene details page in the UCSC Genome Browser.
Further reading
- Lee FJ, Moss J, Vaughan M (1992). "Human and Giardia ADP-ribosylation factors (ARFs) complement ARF function in Saccharomyces cerevisiae". J. Biol. Chem. 267 (34): 24441–5. PMID 1447192.
- Stearns T, Willingham MC, Botstein D, Kahn RA (1990). "ADP-ribosylation factor is functionally and physically associated with the Golgi complex". Proc. Natl. Acad. Sci. U.S.A. 87 (3): 1238–42. Bibcode:1990PNAS...87.1238S. doi:10.1073/pnas.87.3.1238. PMC 53446. PMID 2105501.
- Orcl L, Palmer DJ, Amherdt M, Rothman JE (1993). "Coated vesicle assembly in the Golgi requires only coatomer and ARF proteins from the cytosol". Nature. 364 (6439): 732–4. Bibcode:1993Natur.364..732O. doi:10.1038/364732a0. PMID 8355790.
- Helms JB, Palmer DJ, Rothman JE (1993). "Two distinct populations of ARF bound to Golgi membranes". J. Cell Biol. 121 (4): 751–60. doi:10.1083/jcb.121.4.751. PMC 2119793. PMID 8491770.
- Kanoh H, Williger BT, Exton JH (1997). "Arfaptin 1, a putative cytosolic target protein of ADP-ribosylation factor, is recruited to Golgi membranes". J. Biol. Chem. 272 (9): 5421–9. doi:10.1074/jbc.272.9.5421. PMID 9038142.
- McGuire RE, Daiger SP, Green ED (1997). "Localization and characterization of the human ADP-ribosylation factor 5 (ARF5) gene". Genomics. 41 (3): 481–4. doi:10.1006/geno.1997.4689. PMID 9169151.
- Andreev J, Simon JP, Sabatini DD, et al. (1999). "Identification of a New Pyk2 Target Protein with Arf-GAP Activity". Mol. Cell. Biol. 19 (3): 2338–50. doi:10.1128/MCB.19.3.2338. PMC 84026. PMID 10022920.
- Honda A, Nogami M, Yokozeki T, et al. (1999). "Phosphatidylinositol 4-phosphate 5-kinase alpha is a downstream effector of the small G protein ARF6 in membrane ruffle formation". Cell. 99 (5): 521–32. doi:10.1016/S0092-8674(00)81540-8. PMID 10589680.
- Shin OH, Ross AH, Mihai I, Exton JH (2000). "Identification of arfophilin, a target protein for GTP-bound class II ADP-ribosylation factors". J. Biol. Chem. 274 (51): 36609–15. doi:10.1074/jbc.274.51.36609. PMID 10593962.
- Kondo A, Hashimoto S, Yano H, et al. (2000). "A New Paxillin-binding Protein, PAG3/Papα/KIAA0400, Bearing an ADP-Ribosylation Factor GTPase-activating Protein Activity, Is Involved in Paxillin Recruitment to Focal Adhesions and Cell Migration". Mol. Biol. Cell. 11 (4): 1315–27. doi:10.1091/mbc.11.4.1315. PMC 14849. PMID 10749932.
- Nevrivy DJ, Peterson VJ, Avram D, et al. (2000). "Interaction of GRASP, a protein encoded by a novel retinoic acid-induced gene, with members of the cytohesin family of guanine nucleotide exchange factors". J. Biol. Chem. 275 (22): 16827–36. doi:10.1074/jbc.275.22.16827. PMID 10828067.
- Shin OH, Couvillon AD, Exton JH (2001). "Arfophilin is a common target of both class II and class III ADP-ribosylation factors". Biochemistry. 40 (36): 10846–52. doi:10.1021/bi0107391. PMID 11535061.
- Austin C, Boehm M, Tooze SA (2002). "Site-specific cross-linking reveals a differential direct interaction of class 1, 2, and 3 ADP-ribosylation factors with adaptor protein complexes 1 and 3". Biochemistry. 41 (14): 4669–77. doi:10.1021/bi016064j. PMID 11926829.
- Strausberg RL, Feingold EA, Grouse LH, et al. (2003). "Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences". Proc. Natl. Acad. Sci. U.S.A. 99 (26): 16899–903. Bibcode:2002PNAS...9916899M. doi:10.1073/pnas.242603899. PMC 139241. PMID 12477932.
- Scherer SW, Cheung J, MacDonald JR, et al. (2003). "Human Chromosome 7: DNA Sequence and Biology". Science. 300 (5620): 767–72. Bibcode:2003Sci...300..767S. doi:10.1126/science.1083423. PMC 2882961. PMID 12690205.
- Gerhard DS, Wagner L, Feingold EA, et al. (2004). "The Status, Quality, and Expansion of the NIH Full-Length cDNA Project: The Mammalian Gene Collection (MGC)". Genome Res. 14 (10B): 2121–7. doi:10.1101/gr.2596504. PMC 528928. PMID 15489334.
- Rual JF, Venkatesan K, Hao T, et al. (2005). "Towards a proteome-scale map of the human protein-protein interaction network". Nature. 437 (7062): 1173–8. Bibcode:2005Natur.437.1173R. doi:10.1038/nature04209. PMID 16189514.