Isovaleraldehyde
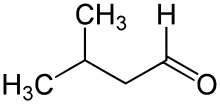
Isovaleraldehyde organic compound, also known as 3-methylbutanal, with the formula (CH3)2CHCH2CHO. It is an aldehyde, a colorless liquid at STP,[1] and found in low concentrations in many types of food.[2] It can be produced commercially and is used as a reagent for the production of pharmaceuticals and pesticides.[3]
 | |
| Names | |
|---|---|
| IUPAC name
3-methylbutyraldehyde | |
| Systematic IUPAC name
3-Methylbutanal | |
| Other names
Isovaleral, Isovaleric Aldehyde | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.008.811 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C5H10O | |
| Molar mass | 86.13[1] |
| Appearance | Colorless Liquid[1] |
| Density | 0.785 g/mL at 20 °C[1] |
| Melting point | −51 °C (−60 °F; 222 K)[1] |
| Boiling point | 92 °C (198 °F; 365 K)[1] |
| Soluble in alcohol and ether, slightly soluble in water[1] | |
| -57.5·10−6 cm3/mol | |
| Hazards | |
| Main hazards | Combustible[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Synthesis
Synthetic routes for the production of isovaleraldehyde vary. One method is by the hydroformylation of isobutene:
- CH3CH3CCH2 + H2 + CO → (CH3)2CHCH2CHO
A small amount of 2,2-dimethylpropanal side product is also generated.[3] Another method of production involves the isomerization of 3-methyl-3-butene-1-ol using CuO-ZnO as a catalyst. A mixture of 3-methyl-3-butene-1-ol and 3-methyl-2-butene-1-ol may also be used. These starting materials are obtained from a reaction between isobutene and formaldehyde:[3]
- CH3CH3CCH2 + CH2O → (CH3)2CHCH2CHO
Finally, in beer the compound is produced via a reaction between the amino acid leucine and reductones in the malt.[4]
Occurrences and uses
As it stems from leucine, the occurrence of isovaleraldehyde is not limited to beer; the compound has found to be a flavor component in many different types of foods. It is described as having a malty flavor and has been found in such foods as beer, cheese, coffee, chicken, fish, chocolate, olive oil, and tea.[2][5]
The compound is used as a reactant in the synthesis of a number of compounds. Notably it is used to synthesize 2,3-dimethyl-2-butene, and is then converted to 2,3-dimethylbutane-2,3-diol and methyltert-butylketone, better known as pinacolone. Pinacolone itself is then used in synthesis for number of pesticides. Additionally, a range of pharmaceuticals, such as butizide, are synthesized from isovaleraldehyde and its corresponding acid.[3]
References
- Lewis, R.J. Sr.; Hawley's Condensed Chemical Dictionary 15th Edition. John Wiley & Sons, Inc. New York, NY 2007., p. 719
- Cserháti, T. and Forgács, E. Encyclopedia of Food Sciences and Nutrition (Second Edition): Flavor (Flavour) Compounds | Structures and Characteristics, Elsevier Science Ltd., 2003, Pg. 2509-2517
- Kohlpaintner, C. Ullmann's Encyclopedia of Industrial Chemistry: Aldehydes, Aliphatic, Wiley-VCH, 2000, Pg. 9 doi:10.1002/14356007.a01_321.pub3
- Bamforth, C.W. Encyclopedia of Food Sciences and Nutrition (Second Edition) | Chemistry of Brewing, 2003, Pg. 440-447
- Owuor, P.O. Encyclopedia of Food Sciences and Nutrition (Second Edition) TEA | Analysis and Tasting, 2003, Pg. 5757-5762