3-Hydroxybenzaldehyde
3-Hydroxybenzaldehyde is one of the three isomers of hydroxybenzaldehyde.
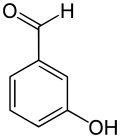 | |
| Names | |
|---|---|
| IUPAC name
3-Hydroxybenzaldehyde | |
| Other names
m-Hydroxybenzaldehyde; m-Formylphenol; 3-Formylphenol | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.002.630 |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C7H6O2 | |
| Molar mass | 122.123 g·mol−1 |
| Appearance | light-tan crystals |
| Melting point | 100 to 103 °C (212 to 217 °F; 373 to 376 K) |
| Boiling point | 191 °C (376 °F; 464 K) (50 mmHg) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Chemistry
It has been prepared from 3-nitrobenzaldehyde in a sequence of nitro group reduction, diazotization of the amine, and hydrolysis.[1][2]
Metabolism
3-hydroxybenzyl-alcohol dehydrogenase is an enzyme that uses 3-hydroxybenzyl alcohol and NADP+ to produce 3-hydroxybenzaldehyde, NADPH and H+.
Uses
3-Hydroxybenzaldehyde is used in the synthesis of monastrol.
gollark: That too.
gollark: There would be exciting safety issues.
gollark: There's more than that.
gollark: So that'll be interesting.
gollark: Oh, Starlink's meant to be happening soon™, isn't it?
See also
- Salicylaldehyde (2-hydroxybenzaldehyde)
- 4-Hydroxybenzaldehyde
References
- m-HYDROXYBENZALDEHYDE, Organic Syntheses, Coll. Vol. 3, p.453 (1955); Vol. 25, p.55 (1945)
- m-METHOXYBENZALDEHYDE Archived 2012-10-04 at the Wayback Machine, Organic Syntheses, Coll. Vol. 3, p.564 (1955); Vol. 29, p.63 (1949)
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.