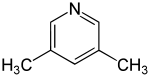
3,5-Lutidine
3,5-Lutidine is a heterocyclic organic compound with the formula (CH3)2C5H3N. It is one of several dimethyl-substituted derivatives of pyridine, all of which are referred to as lutidines. It is a colorless liquid with mildly basic properties and a pungent, noxious odor. The compound is a precursor to the drug omeprazole.
 | |
| Names | |
|---|---|
| Preferred IUPAC name
3,5-Dimethylpyridine | |
| Other names
3,5-Lutidine | |
| Identifiers | |
3D model (JSmol) |
|
| 105682 | |
| ChemSpider | |
| ECHA InfoCard | 100.008.827 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C7H9N | |
| Molar mass | 107.156 g·mol−1 |
| Appearance | Clear oily liquid |
| Density | 0.944 g/cm3 |
| Melting point | −6.5 °C (20.3 °F; 266.6 K) |
| Boiling point | 171.9 °C (341.4 °F; 445.0 K) |
| Acidity (pKa) | 6.15[1] |
| -71.72·10−6 cm3/mol | |
| Hazards | |
| GHS pictograms |     |
| GHS Signal word | Danger |
GHS hazard statements |
H226, H301, H302, H311, H312, H314, H315, H318, H331, H332, H335 |
| P210, P233, P240, P241, P242, P243, P260, P261, P264, P270, P271, P280, P301+310, P301+312, P301+330+331, P302+352, P303+361+353, P304+312, P304+340, P305+351+338, P310, P311, P312, P321, P322 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
It is produced industrially by condensation of acrolein, ammonia, and formaldehyde.[1]
Biodegradation
The biodegradation of pyridines proceeds via multiple pathways.[2] Although pyridine is an excellent source of carbon, nitrogen, and energy for certain microorganisms, methylation significantly retards degradation of the pyridine ring.[3][4]
Safety
The LD50 is 200 mg/kg (oral, rats).
gollark: ddg!eso Brotlipython
gollark: I have PRODUCED ESOLANG:
gollark: !quote 740909409378762812
gollark: !quote 741570646760488980
gollark: A beesolang, if you will.
See also
References
- Shimizu, Shinkichi; Watanabe, Nanao; Kataoka, Toshiaki; Shoji, Takayuki; Abe, Nobuyuki; Morishita, Sinji; Ichimura, Hisao (2007). "Pyridine and Pyridine Derivatives". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a22_399.
- Philipp, Bodo; Hoff, Malte; Germa, Florence; Schink, Bernhard; Beimborn, Dieter; Mersch-Sundermann, Volker (2007). "Biochemical Interpretation of Quantitative Structure-Activity Relationships (QSAR) for Biodegradation of N-Heterocycles: A Complementary Approach to Predict Biodegradability". Environmental Science & Technology. 41 (4): 1390–8. doi:10.1021/es061505d. PMID 17593747.
- Sims, G. K.; Sommers, L.E. (1985). "Degradation of pyridine derivatives in soil". Journal of Environmental Quality. 14 (4): 580–584. doi:10.2134/jeq1985.00472425001400040022x.
- Sims, G. K.; Sommers, L.E. (1986). "Biodegradation of Pyridine Derivatives in Soil Suspensions". Environmental Toxicology and Chemistry. 5 (6): 503–509. doi:10.1002/etc.5620050601.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.