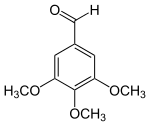
3,4,5-Trimethoxybenzaldehyde
3,4,5-Trimethoxybenzaldehyde is an organic compound. Within this class of compounds the chemical is categorized as a trisubstituted aromatic aldehyde.
 | |
| Names | |
|---|---|
| Preferred IUPAC name
3,4,5-Trimethoxybenzaldehyde | |
| Systematic IUPAC name
3,4,5-Trimethoxybenzenecarbaldehyde | |
| Other names
3,4,5-Trimethoxy-benzaldehyde 3,4,5-Trimethoxy benzaldehyde | |
| Identifiers | |
3D model (JSmol) |
|
| 395163 | |
| ChemSpider | |
| ECHA InfoCard | 100.001.547 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C10H12O4 | |
| Molar mass | 196.202 g·mol−1 |
| Appearance | Light yellow solid |
| Density | 1.367 g/cm3 |
| Melting point | 73 °C (163 °F; 346 K) |
| Boiling point | 337.6 °C (639.7 °F; 610.8 K) |
| Slightly soluble | |
| Solubility | Methanol: 0.1 g/mL |
| Hazards | |
| Safety data sheet | MSDS at Sigma Aldrich |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Uses
3,4,5-Trimethoxybenzaldehyde can be used as an intermediate in the synthesis of some pharmaceutical drugs including trimethoprim,[1][2] cintriamide, roletamide, trimethoquinol (aka tretoquinol), and trimazosin as well as some psychedelic phenethylamines.[3][4][5]
Preparation
Industrial scale
For industrial applications the chemical is synthesized from p-cresol using aromatic substitution with bromine followed by nucleophilic substitution with sodium methoxide.[1] Oxidation of the methyl group to an aldehyde can occur via various synthetic methods.
Laboratory scale
At the laboratory scale the chemical is conveniently synthesized from vanillin[6] or from Eudesmic acid's Acyl chloride via Rosenmund reduction.[7]
References
- Asim Kumar Mukhopadhyay (2004). Industrial Chemical Cresols and Downstream Derivatives. New York: CRC Press. p. 81. ISBN 9780203997413. Retrieved 2 June 2014.
- Stenbuck, P.; Hood, H. M.; 1962, U.S. Patent 3,049,544
- Kindler, Karl & Peschke, Wilhelm (1932). "Über neue und über verbesserte Wege zum Aufbau von pharmakologisch wichtigen Aminen VI. Über Synthesen des Meskalins". Archiv der Pharmazie. 270 (7): 410–413. doi:10.1002/ardp.19322700709.
- Benington, Fred & Morin, Richard (1951). "An Improved Synthesis of Mescaline". Journal of the American Chemical Society. 73 (3): 1353–1353. doi:10.1021/ja01147a505.
- Shulgin, Alexander & Shulgin, Ann (1991). PiHKAL: A Chemical Love Story. Lafayette, CA: Transform Press. ISBN 9780963009609.
- Manchanda Percy; Belicaa, Peter & Wonga, Harry (1990). "Synthesis of 3,4,5-Trimethoxybenzaldehyde". Synthetic Communications. 20 (17): 2659–2666. doi:10.1080/00397919008051474.
- Rachlin, A.; Gurien, H. & Wagner, D. (1971). "Aldehydes from Acid Chlorides by modified Rosenmund Reduction: 3,4,5-Trimethoxybenzaldehyde". Organic Syntheses. 51: 8. doi:10.15227/orgsyn.051.0008.