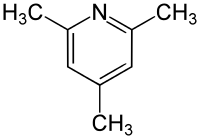
2,4,6-Trimethylpyridine
2,4,6-Trimethylpyridine (2,4,6-collidine) is an organic compound which belongs to the heterocycles (more precisely, heteroaromatics). It consists of a pyridine ring substituted with three methyl groups. It belongs to the substance group of the collidines, a group of six constitutional isomers. 2,4,6-trimethylpyridine is the most well-known isomer of this group.
 | |
| Identifiers | |
|---|---|
| ECHA InfoCard | 100.003.286 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| Properties | |
| C8H11N | |
| Molar mass | 121,18 g·mol−1 |
| Appearance | colourless liquid with pyridine-like smell[1] |
| Boiling point | 171–172 °C[1] |
| 35 g·l−1 (20 °C)[1] | |
| Vapor pressure | 4 hPa (20 °C)[1] |
| Acidity (pKa) | 7,43 (25 °C)[2] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Properties
The compound has a refractive index of 1,4959 (25 °C).[3]
Preparation
2,4,6-Trimethylpyridine was isolated from bone oil in 1854.[4][5] A synthesis can be carried out analogously to the Hantzsch's dihydropyridine synthesis from ethyl acetoacetate (as β-ketocarbonyl compound), acetaldehyde and ammonia in the ratio 2: 1: 1.[6]
Use
By oxidation of the methyl groups with potassium permanganate collidinic acid is obtained.[7]
2,4,6-Trimethylpyridine is used in organic syntheses (for example, for dehydrohalogenation[8]), by binding the formed hydrogen halides.
See also
- 2,6-Dimethylpyridine (lutidine)
References
- Record of 2,4,6-Trimethylpyridin in the GESTIS Substance Database of the Institute for Occupational Safety and Health, accessed on 27. Juli 2017.
- Zvi Rappoport: CRC Handbook of Tables for Organic Compound Identification, Third Edition, CRC Press, Boca Raton, Florida, 1984, ISBN 0-8493-0303-6, S. 438.
- David R. Lide (ed.). Physical Constants of Organic Compounds. CRC Handbook of Chemistry and Physics (90th ed.). Boca Raton, FL: CRC Press/Taylor and Francis. pp. 3–510. (Internet-Version: 2010).
- Collidin. In: Heinrich August Pierer, Julius Löbe (Hrsg.): Universal-Lexikon der Gegenwart und Vergangenheit. 4. Auflage. Band 4. Altenburg 1858, S. 262 (zeno.org).
- Friedrich Auerbach: „Ueber ein neues Collidin“, in: Berichte der deutschen chemischen Gesellschaft, 1892, 25 (2), S. 3485–3490 (doi:10.1002/cber.189202502219).
- T. Laue, A. Plagens: Namen- und Schlagwort-Reaktionen der Organischen Chemie, 5. Auflage, Teubner Verlag, Wiesbaden 2006, ISBN 3-8351-0091-2, S. 172.
- Hong-Lin Zhu; Wei Xu; Jian-Fei Wang; Yue-Qing Zheng (2012), "Synthesis, crystal structures and properties of two supramolecular polymers constructed by lanthanide with pyridine-2,4,6-tricarboxylic acid", Synthetic Metals (in German), 162 (13–14), pp. 1327–1334, doi:10.1016/j.synthmet.2012.05.016
- Entry on Kollidin. at: Römpp Online. Georg Thieme Verlag, retrieved 27. Juli 2017.